 |
 |
 |
| |
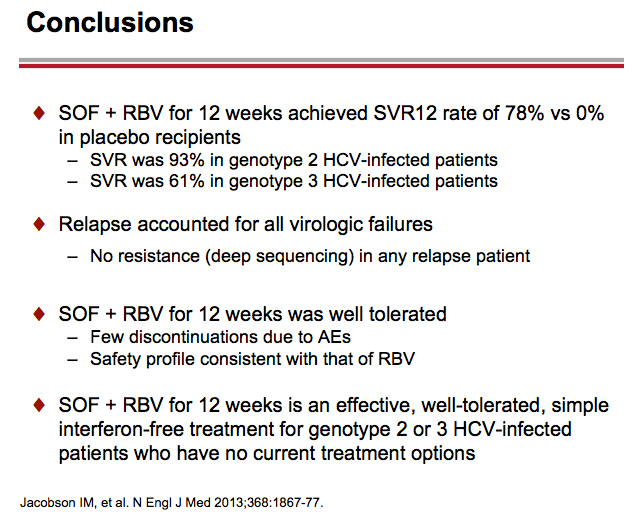
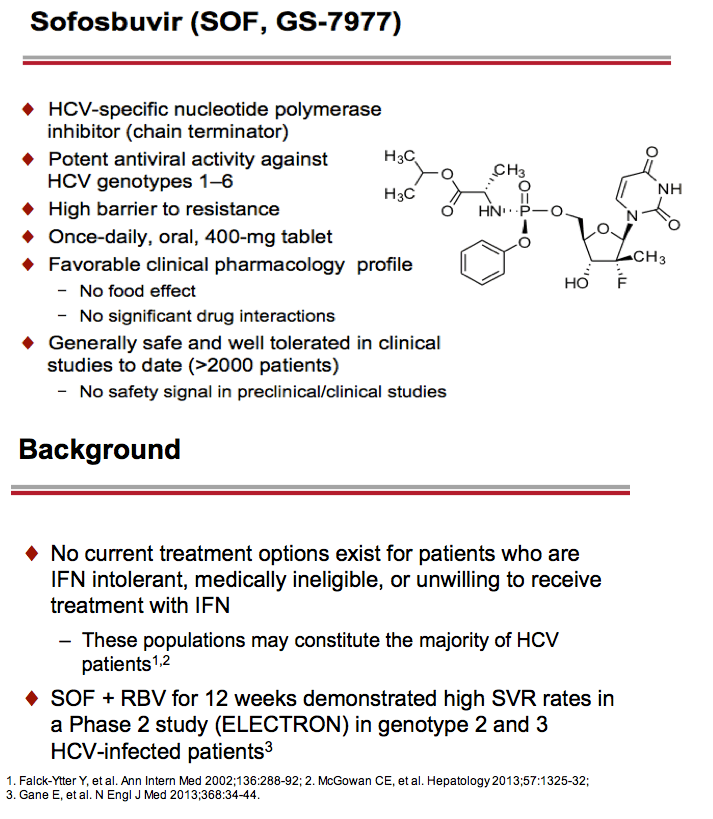
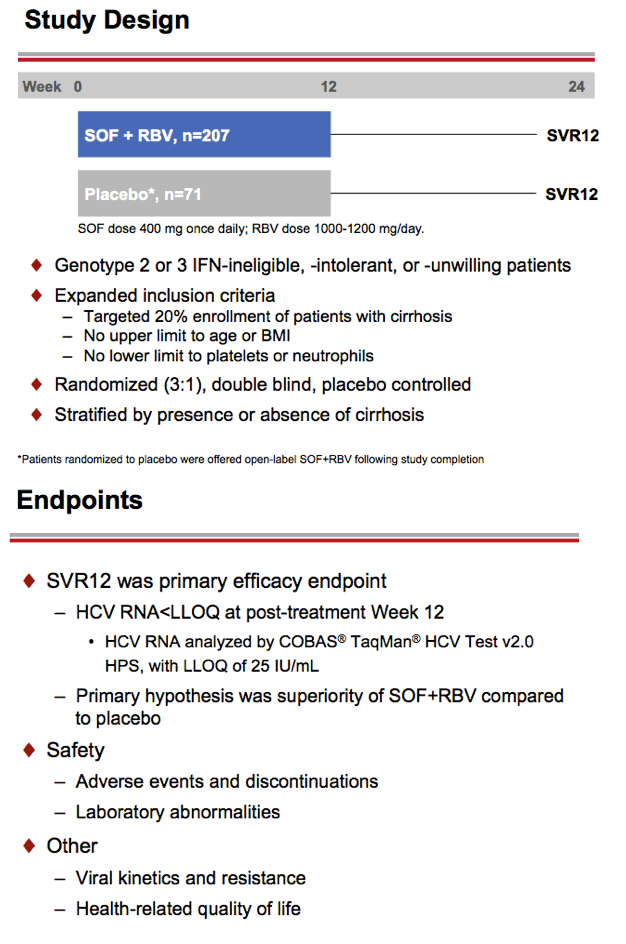
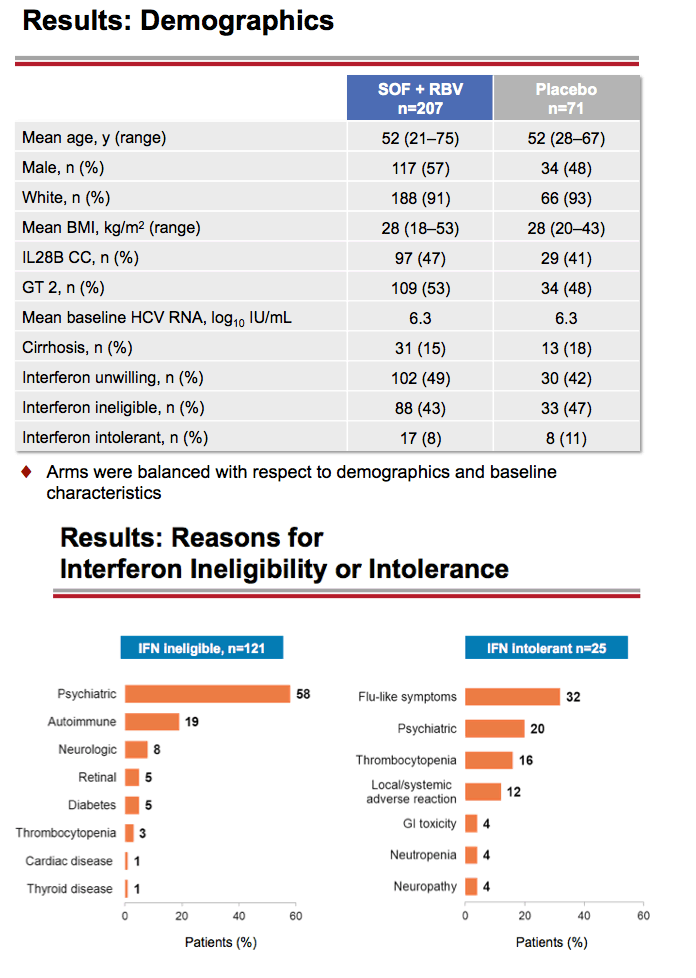
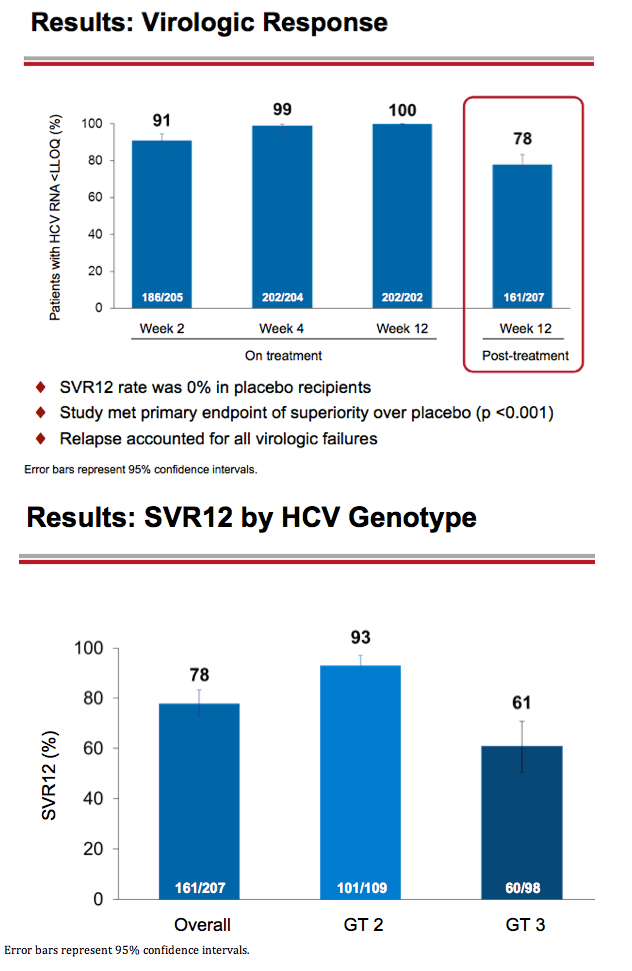
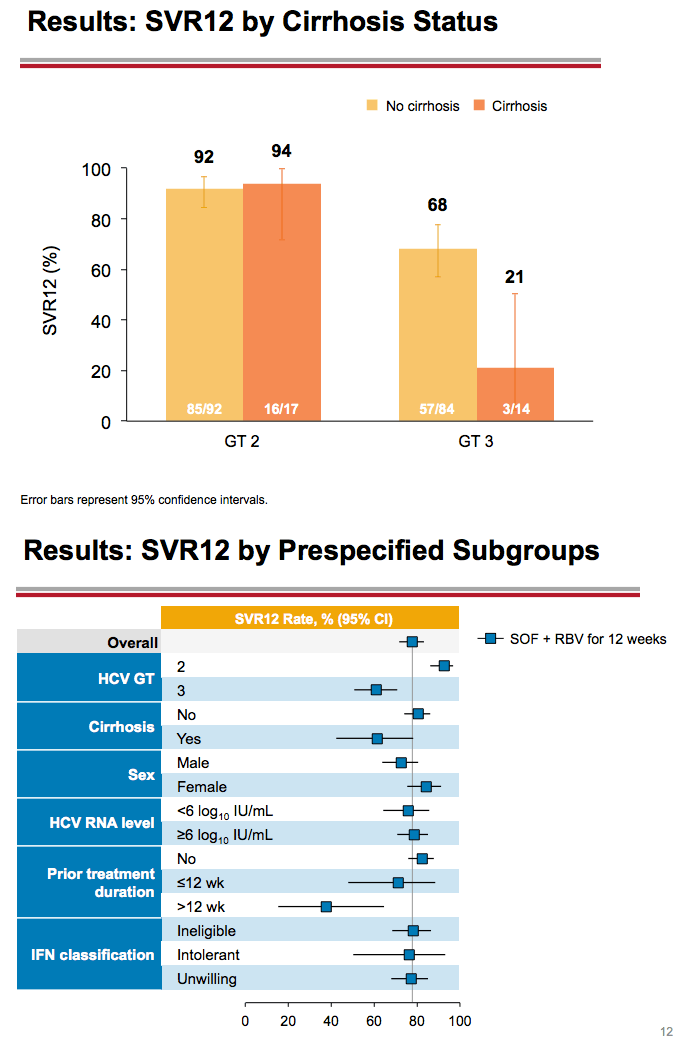
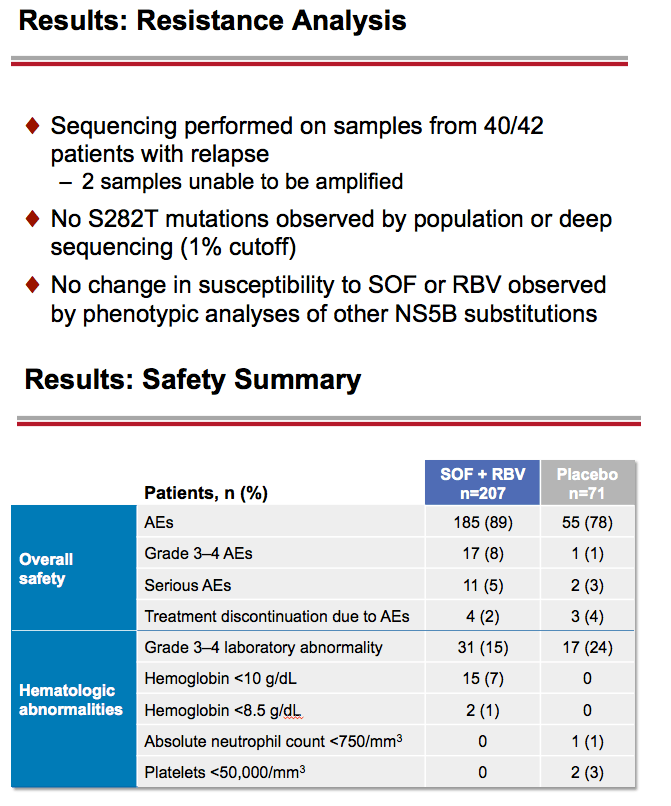
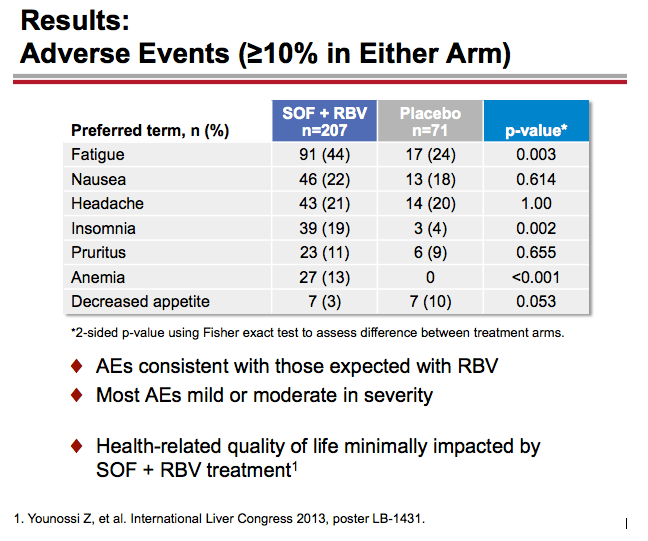
Treatment with Sofosbuvir+Ribavirin for 12 Weeks Achieves SVR12 of 78% in GT2/3 Interferon-Ineligible, -Intolerant, or -Unwilling Patients: The POSITRON Trial
|
| |
| |
Reported by Jules Levin
APASL 2013 June 6-10 Singapore
Stephen Pianko1, Ira M. Jacobson2, Mark Sulkowski3, David R. Nelson4, Evguenia Svarovskaia5, Di An5, John McNally5,
Diana M. Brainard5, William T. Symonds5, John G. McHutchison5, Kris V. Kowdley6, Eric M. Yoshida7
1Monash Medical Centre and Monash University, Melbourne, Victoria, Australia; 2Weill Cornell Medical College, New York, NY, USA; 3Johns Hopkins University School of Medicine, Baltimore, MD, USA; 4University of Florida, Gainesville, FL, USA; 5Gilead Sciences, Inc., Foster City, CA, USA; 6Digestive Disease Institute, Virginia Mason Medical Center, Seattle, WA, USA; 7University of British Columbia, Vancouver, Canada
|
| |
|
 |
 |
|
|