 |
 |
 |
| |
Dolutegravir Has No Effect on the Pharmacokinetics of Methadone or Oral Contraceptives With Norgestimate and Ethinyl Estradiol
|
| |
| |
Reported by Jules Levin
CROI 2013
Ivy Song,1 Stephen Mark,1 Julie Borland,1 Shuguang Chen,1 Toshihiro Wajima,2 Amanda Peppercorn,1 Stephen Piscitelli1*
1GlaxoSmithKline, Research Triangle Park, NC; Mississauga, ON, Canada; 2Shionogi & Co., Ltd., Osaka, Japan
Abstract
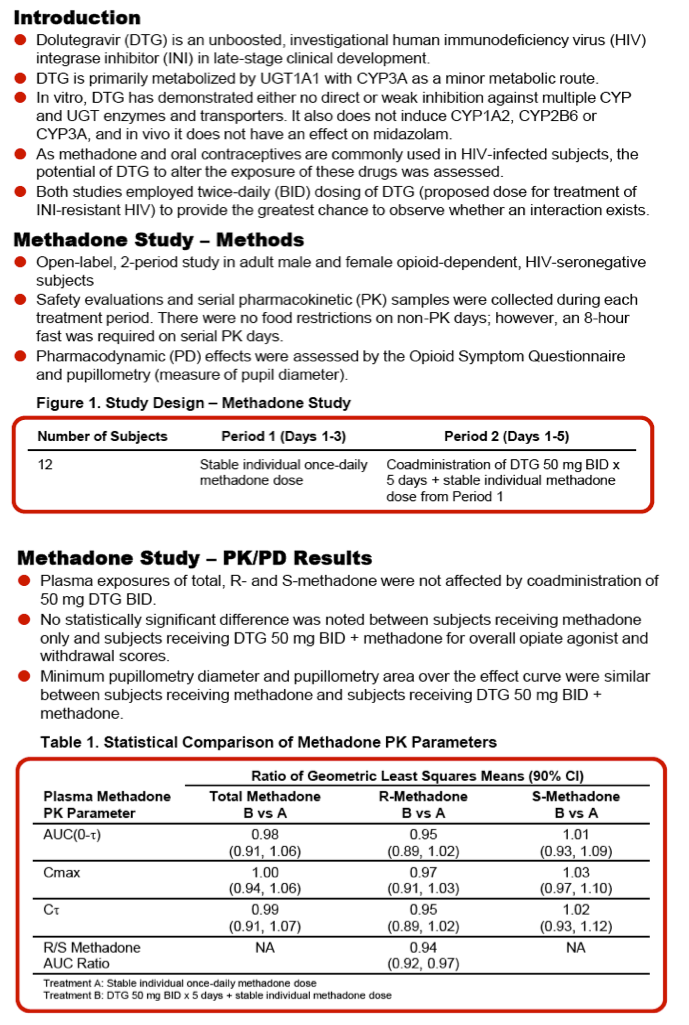
Background: Dolutegravir (DTG) is an unboosted HIV integrase inhibitor that has no significant cytochrome P450 enzyme or UDP-glucuronosyltransferase inhibition or induction. Although the drug interaction potential of DTG is low, studies were performed to confirm that the highest clinical dose of DTG (50 mg twice daily [BID]) has no impact on the pharmacokinetics (PK) and pharmacodynamics (PD) of methadone or a common oral contraceptive.
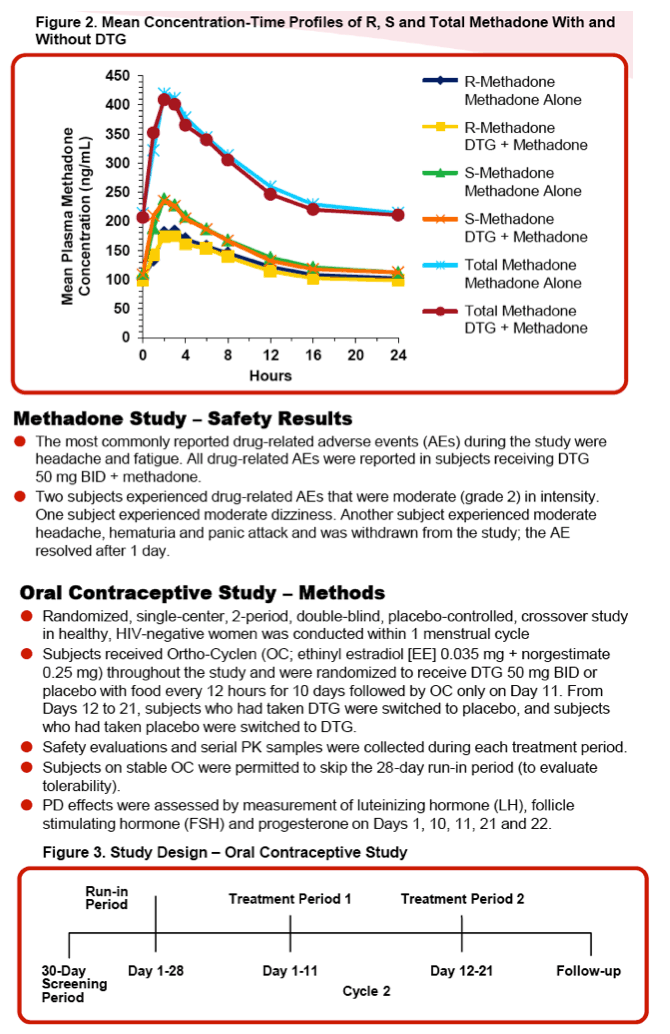
Methods: Study 1 was an open-label, 2-period study in adult opiate-dependent, HIV-seronegative subjects. Subjects received their current individual doses of methadone once daily for 3 days (Period 1) followed by DTG 50 mg BID for 5 days with stable methadone therapy (Period 2). Serial PK samples for R- and S-methadone were collected on Period 1 Day 3 and Period 2 Day 5 after an 8-hour fast. Pharmacodynamic measures (pupillometry and overall opiate agonist and withdrawal scores) and safety assessments were obtained throughout the study.
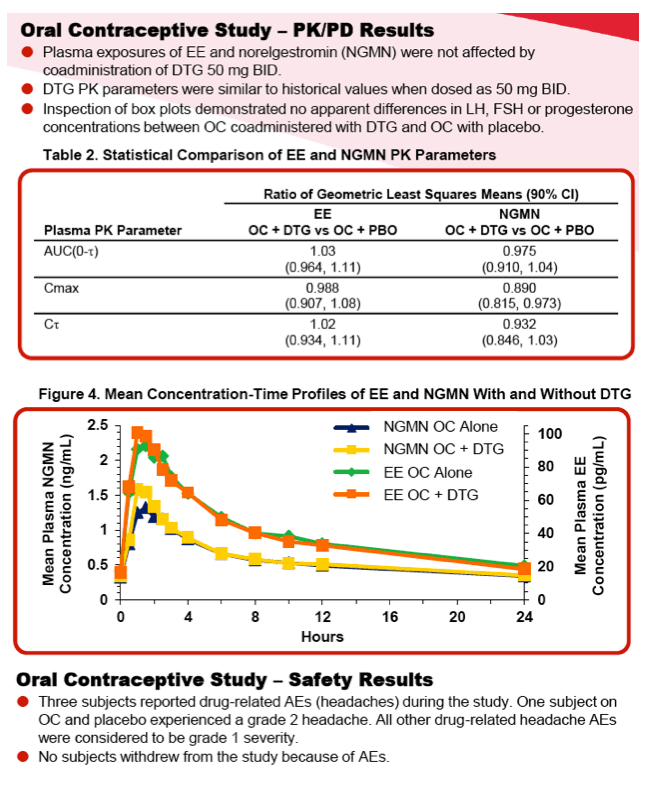
Study 2 was a randomized, 2-period, double-blind, placebo-controlled, crossover study conducted within a single menstrual cycle. Healthy female subjects received Ortho-Cyclen® (ethinyl estradiol [EE] 0.035 mg and norgestimate 0.25 mg) throughout the study and were randomized to receive DTG 50 mg BID or placebo with food every 12 hours for 10 days followed by Ortho-Cyclen only on Day 11. From Days 12 to 21, subjects who had taken DTG were switched to placebo, and subjects who had taken placebo were switched to DTG, which subjects took every 12 hours with food. Serial PK samples and safety assessments were obtained throughout the study. To assess PD, luteinizing hormone, follicle stimulating hormone and progesterone levels were collected on Days 1, 10, 11, 21 and 22. For both studies, noncompartmental PK analysis was performed and geometric least squares (GLS) mean ratios and 90% CI were generated by the mixed effect model for within-subject treatment comparison.
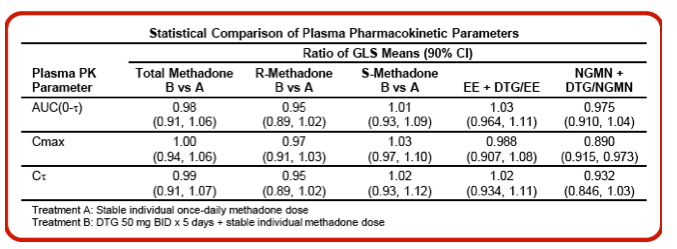
Results: The PK of methadone, EE and norelgestromin (NGMN) were not altered by concomitant DTG and there were no clinically relevant differences in PD measures. All adverse events (AEs) were mild or moderate in severity. No death, serious AE or grade 3/4 AE occurred. No clinically significant changes in laboratory values, vital signs or ECGs were observed.
Conclusions: No dose adjustment in methadone is required when given in combination with DTG, and oral contraceptives can be coadministered with DTG 50 mg once or twice daily.
|
| |
|
 |
 |
|
|