 |
 |
 |
| |
Clinical Pharmacology at CROI 2013
|
| |
| |
Courtney V. Fletcher, Pharm.D.
Dean and Professor
College of Pharmacy
University of Nebraska Medical Center
986000 Nebraska Medical Center
Omaha, NE 68198
The 20th Conference on Retroviruses and Opportunistic Infections (CROI) was held in Atlanta, GA, from March 3 to March 6, 2013. CROI continues to be the premier HIV-focused scientific meeting. In this report I will highlight abstracts focused on pharmacologic issues I think are of broad interest or might benefit from some expert clarification. I will discuss abstracts in five broad categories: the therapy of HIV infection, PrEP, advances in treatment of the TB-HIV coinfected person, new drugs for treatment of HCV, and compartment/reservoir/cure research.
Abbreviations
%CV, percent coefficient of variation
ABC, abacavir
ACTG, adult AIDS clinical trials group
APV, amprenavir
ARV, antiretroviral drug
ART, antiretroviral drug therapy
AUC, area under the concentration-time curve
ATV, atazanavir
BOC, boceprevir
C12, drug concentration at 12 hours post dose
Cmax, maximum drug concentration
Cmin, minimum drug concentration
CVC, cenicriviroc
CNS, central nervous system
COBI, cobicistat
CSF, cerebrospinal fluid
CVF, Cervicovaginal fluid
Ctrough, concentration immediately before the next dose
CYP, cytochrome P450 drug metabolizing enzymes
DBS, dried blood spot
DHHS, Department of Health and Human Services
DSMB, data safety monitoring board
DTG, dolutegravir
DRV, darunavir
ddI, didanosine
EFV, efavirenz
EVG, elvitegravir
FDV, faldaprevir
FTC, emtricitabine
ETR, etravirine
fAPV, fosamprenavir
GMR, geometric means ratio
HAND, HIV-associated neurocognitive disorders
HDAc, histone deacetylase
IC50, concentration of drug required to inhibit viral replication in vitro by 50%
IC90, concentration of drug required to inhibit viral replication in vitro by 90%
IDV, indinavir
IM, intramuscular
IQ, inhibitory quotient
IVR, intra-vaginal ring
3TC, lamivudine
LDV, ledipasvir
LPV, lopinavir
MVC, maraviroc
NVP, nevirapine
NRTI, nucleoside reverse transcriptase inhibitor
NNRTI, non-nucleoside reverse transcriptase inhibitor
PACTG, pediatric AIDS clinical trials group
PBMCs, peripheral blood mononuclear cells
PD, pharmacodynamic
PG, pharmacogenetics/pharmacogenomics
PK, pharmacokinetic
PI, inhibitor of HIV protease
PrEP, pre-exposure prophylaxis
r or RTV, ritonavir
RAL, raltegravir
RBT, rifabutin
RBV, ribavirin
RPT, rifapentine
RIF, rifampin
RPV, rilpivirine
SQV, saquinavir
SC, subcutaneous
SOF, sofosbuvir
TAF, tenofovir alafenamide fumarate
TDF, tenofovir disoproxil fumarate
TFV, tenofovir
TVR, telaprevir
TDM, therapeutic drug monitoring
TPV, tipranavir
TB, tuberculosis
ZDV, zidovudine
I. The Pharmacotherapy of HIV Infection
1. Dolutegravir. The pharmacologic properties of dolutegravir (DTG) were the topic of several presentations. Poster 178LB investigated concentrations in the CSF, and found that CSF concentrations were the same as unbound plasma concentrations in HIV-infected, ARV-naïve subjects, and that the CSF concentrations were ≥ IC50 for the drug against wild type virus (0.2 ng/mL). Importantly, a regimen of DTG+ABC+3TC achieved a CSF viral load reduction at 16 weeks similar to that in plasma (both approximately -3 log). Poster 531 assessed DTG concentrations in colorectal tissue, as well as in male and female reproductive tracts. Low DTG concentrations were observed in reproductive tract secretions of both men and women. Interestingly, however, tissue concentrations in female reproductive tract and rectum in the same subjects were above the IC90 for the drug. Poster 535 assessed the pharmacokinetics of methadone and oral contraceptives when given with DTG. No significant PK interaction was observed with oral contraceptives or methadone, which provides support that coadministration is acceptable. However, clinicians must always consider all drugs a patient is taking before making a final determination on allowable concomitant medications. There has been a concerted effort to obtain a very complete pharmacologic profile of DTG during drug development - I believe this information has and will play a key role in judgments about its safety and efficacy and future role in the pharmacotherapy of HIV infection.
CROI: Distribution and Antiviral Activity in Cerebrospinal Fluid (CSF) of the Integrase Inhibitor, Dolutegravir (DTG): ING116070 Week 16 Results - (03/06/13)
CROI: Dolutegravir Treatment Response and Safety by Key Subgroups in Treatment Naive HIV Infected Individuals - (03/16/13)
CROI: Dolutegravir (DTG) Versus Raltegravir (RAL) in ART-Experienced, Integrase-Naive Subjects: 24-Week Interim Results From SAILING (ING111762) - (03/06/13)
2. The new NNRTI from Merck. Poster 527 assessed the PK of Merck's new investigational NNRTI, MK-1439 in healthy subjects. The PK profile of the drug is conducive to daily dosing (elimination half-life of 10-16 hrs). A drug interaction study with midazolam showed that MK-1439 was not an inducer or inhibitor of CYP3A. What we haven't seen yet is whether MK-1439 will be susceptible to (e.g. a victim of) drug interactions. Presentation 100 described a double-blinded trial comparing both 25mg and 200mg daily doses of MK-1439 with placebo for 1 week in HIV-infected individuals. No serious adverse effects judged to be associated with MK-1439 were observed. In this monotherapy trial, robust antiviral effects (1.37 log decline with 25mg, 1.26 log decline with 200mg, as compared with placebo) were seen. Importantly, no evidence for emergence of viral resistance was seen in this weeklong trial. Additional trials for this drug are ongoing, and it will be worth following in the future.
CROI: Safety and Antiviral Activity of MK-1439, a Novel Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI), In Treatment-Naïve HIV-Infected Patients - (03/05/13)
3. Cenicriviroc. Week 24 analysis of cenicriviroc (CVC) was presented in abstract 106LB. CVC is an investigational once daily CCR5 and CCR2 antagonist. Two different doses of CVC (100 mg and 200 mg once daily) were compared with EFV, both in combination with FTC/TDF in treatment-naïve adults. The percentages of individuals experiencing virologic success were similar between the CVC and EFV groups. However, virologic non-response was higher with CVC than with EFV. Fewer CVC recipients discontinued therapy due to adverse effects. CVC is moving forward into phase 3 trials; I think the optimal dose is still to be determined.
CROI: Week 24 Primary Analysis of Cenicriviroc vs Efavirenz, in Combination with FTC/TDF, in Treatment-naïve HIV-1 Infected Adults with CCR5-tropic virus - (03/08/13)
4. The renal safety of TDF. At CROI and in the recent literature, there have been several reports regarding the renal safety of TDF. At CROI 2013, in a cohort of 101 HIV-infected women, RTV use, increasing age, decreasing BMI and pre-existing renal impairment were associated with higher tenofovir (TFV) plasma concentrations (Abstract 522). In a cohort of 100 HIV-infected persons with baseline creatinine clearance > 80 mls/min, a higher serum creatinine and lower body weight were associated with increased TFV plasma concentrations (Abstract 523). Females weighing < 50 kg had the highest TFV concentrations, while females ≥ 50 kg and males either < 65kg or ≥ 65 kg had comparable TFV concentrations. Patients (N=26) who experienced TDF-associated toxicities (renal and/or bone) were older and had significantly higher TFV concentrations (145 vs. 101 ng/mL). Women experiencing these adverse events had higher TFV concentrations than those who did not (173 vs. 106 ng/mL).
(http://www.natap.org/2013/CROI/croi_158.htm)
Poizot-Martin (JAIDS 2013;62:375-80) performed a retrospective analysis of tenofovir (TFV) plasma trough concentrations and the risk of renal impairment in 163 HIV-infected persons. At the time TFV trough concentrations (Ctrough) were measured, these individuals had received a median of 21.1 months of TDF therapy. A significant decrease in renal function (as assessed by estimated glomerular filtration rate [GFR]) was seen in those who had Ctrough > 90 ng/mL (univariate analysis). A multivariate analysis found a Ctrough > 90 ng/mL was significantly associated with a decrease in GFR in only women.
(http://www.natap.org/2013/HIV/022713_01.htm)
D:A:D Study investigators (Ryom L et al, J Infect Dis 2013; 207:1359-69) http://www.natap.org/2013/HIV/040313_02.htm)
have recently reported their evaluation of renal impairment in HIV-infected persons with normal baseline renal function. Among 22,603 persons they found that 468 (2%) developed a GFR <70 mls/min and 131 (0.6%) developed a GFR <60 mls/min, which they defined as moderately severe chronic kidney disease (CKD). Cumulative use of TDF, ATV/RTV, and LPV/RTV were each independent predictors of GFR <70 mls/min; only LPV/RTV was an independent predictor of GFR <60 mls/min (CKD). Among patient characteristics, older age, diabetes, lower CD4 cell count, and female sex were associated with an increased risk for renal impairment. A GFR of 60-70 mls/min was associated with a higher rate of TDF discontinuation, most likely indicating clinicians switched away from TDF use in persons with renal insufficiency - a reassuring finding! (poster: http://www.retroconference.org/2013b/PDFs/810.pdf)
Collectively, what do these reports (and other literature) tell us (also see Fine and Gallant, J Infect Dis 2013;207:1349-51).http://www.natap.org/2013/HIV/040313_01.htm) First, TDF use is associated with a risk of renal insufficiency, but it is not the only antiretroviral drug with this association. Second, concomitant therapies and interactions among agents may increase the risk of renal impairment. One example may be the drug-drug interaction between RTV-boosted PIs and TDF, which increases plasma concentrations of TFV and decreases renal clearance of TFV; greater declines in renal function have been observed with TDF + PI/RTV compared with TDF + NNRTI (J Infect Dis 2008;197:102-8). Third, clinicians need to ensure periodic monitoring of renal function, and if there is a decline in GFR, the dose of all renally excreted drugs needs to be adjusted, and if possible the causative drug discontinued. Finally, the association of TDF therapy, certain TFV plasma concentrations and some patient characteristics (perhaps female sex) with renal insufficiency does provide an argument for therapeutic drug monitoring (TDM) as a potential maneuver to reduce the risk of renal insufficiency. Though I would not argue with clinicians who wish to use TDM in their patients, I would not recommend it routinely. I don't think the plasma concentration associated with an increased risk is defined precisely enough and I'd like some prospective data that provide evidence for a benefit. Another consideration that decreases my enthusiasm is the development of tenofovir alafenamide fumarate (TAF). I think the emerging data indicate, but do not yet prove, that this agent may be a more effective and less toxic substitute for TDF, which could render TDM for TDF not necessary. More on TAF follows.
5. The next generation tenofovir - tenofovir alafenamide fumarate (TAF). Formerly known as GS-7340, TAF received considerable attention at CROI-2013. Posters 529 and 540 examined the effects of TAF on the kidneys. Unlike tenofovir disoproxil fumarate (TDF), TAF does not concentrate in the kidneys. Subjects with severe renal impairment receiving TDF had significantly higher TFV AUC and Cmax (approximately 5.7-fold) as compared with subjects who had normal renal function. In contrast, subjects (with renal impairment) receiving TAF had less than 2-fold increases in AUC and Cmax, which is not considered clinically relevant. These findings suggest TAF may be able to be used in individuals with renal dysfunction without needing to reduce the dose. This suggestion requires a demonstration of safety in HIV-infected persons with renal insufficiency.
Presentation 99LB described a phase 2 trial comparing EVG/COBI/FTC/TDF (QUAD or Stribild®), with the same regimen except that TDF was replaced with TAF. At week 24, the TAF-containing regimen had similar efficacy, and an improved side effect profile than Stribild®; the group receiving TAF experienced no renal side effects, and a significantly smaller decline in bone marrow density. TAF continues to be an exciting new formulation - stay tuned for the phase 3 trials of EVG/COBI/FTC/TAF.
(http://www.natap.org/2013/CROI/croi_23.htm)
II. Pre-Exposure Prophylaxis
1. PrEP - once again, adherence matters. One of the more disappointing results reported at CROI 2013 were from the VOICE study (Abstract 26LB). VOICE study examined PrEP for HIV in a group of predominately young unmarried women in Africa. 5029 participants were randomized to one of: daily oral TDF, oral TDF/FTC, 1% vaginal TFV gel, or placebo. 334 seroconversions occurred within the study; none of the regimens significantly reduced the risk of HIV infection. Tenofovir (TFV) plasma concentrations were sampled within the study population quarterly. TFV was found in a very low percentage of patients randomized to receive study drug. Average detection rates were 28% in patients in the TDF arm, 29% in the TDF/FTC arm and 22% in the TFV gel arm. These results clearly indicate very poor adherence by the study population, and leads to the conclusion that suboptimal adherence explains the lack of protection against acquisition of HIV infection.
The failures of the VOICE trial and the FEM-PrEP trial reported last year at CROI 2012 (abstract 32LB; N Engl J Med. 2012 Aug 2;367(5):411-22) and what we have learned about the determinants of efficacy in CAPRISA 004 and iPrEx collectively reinforce two universally applicable principles of clinical pharmacology: drugs don't work in patients who don't take them (C. Everett Koop) and there is a relationship between the concentration of drug at the site of action and the effect achieved. Before another 5000+ participant pre-exposure clinical trial is launched, and before we learn again that a large number of HIV seroconversions were the result of poor medication adherence, lets go back and learn some basics. Namely, what are the barriers to medication adherence and what strategies are successful in improving and sustaining high levels of adherence?
Staying on the topic of adherence, poster 1006 correlated concentrations of TFV-DP in dried blood spots (DBS) with pharmacy refill history reports as a measure of adherence in HIV-infected women. DBS were collected twice in a group of 25 women approximately 1 week apart. The authors showed the TFV-DP DBS assay was an effective measure of adherence and had a strong correlation with pharmacy refill adherence. This work came from the same group that recently published an elegant analysis of the iPrEx study showing prophylaxis efficacy was strongly correlated with drug adherence rates as measured by intracellular drug levels (see Anderson PL et al, Sci Transl Med, Sept 12, 2012).
2. New formulations to improve PrEP adherence. As disappointing as the VOICE trial data were, there was reason for optimism in attempts to combat the issue of poor adherence rates in PrEP. Oral abstract 24LB presented data on a long acting nano-formulation of GSK744, an integrase inhibitor that is an analog of dolutegravir. The drug was administered via a single intramuscular injection to healthy volunteers and found to have a half-life of 21 to 50 days. This half-life might allow a once monthly or even once quarterly dosing schedule. To assess the efficacy of this long acting GSK744 formulation for PrEP, 8 macaques received intramuscular doses of GSK744 at two time points 4 weeks apart. Dosing started 1 week before intrarectal exposures to SHIV, which were given weekly for up to 8 exposures. All eight of the control macaques receiving placebo became infected with SHIV. None of the 8 treated macaques had detectable virus 3 weeks after the final viral challenge. GSK744, and other long acting ARV formulations may play a key role in minimizing adherence issues facing PrEP regimens. (http://www.natap.org/2013/CROI/croi_38.htm)
Continuing with PrEP, abstract 25LB presented data on a novel intravaginal ring (IVR) composed of polyurethane that releases 2.3mg/day of TDF. Researchers tested the efficacy of the IVR by treating 6 macaque monkeys with IVR's replaced every 28 days and challenged intravaginally with SHIV weekly for 16 weeks. After 16 weeks the IVR treated primates had no incidence of SHIV infection whereas 11 of the 12 control macaques (no IVR treatment) became seropositive. This was the first IVR study to demonstrate 100% protection from SHIV challenges. (http://www.natap.org/2013/CROI/croi_63.htm)
It is essential, I believe, that we have a complete understanding of the PKPD - or the concentrations associated with prevention of transmission for these new PrEP agents and formulations. The success of a PrEP regimen is a yes/no event - and that is a different consequence that not quite achieving the optimal viral load reduction for a new drug to treat established HIV infection.
III. Advances in the Treatment of Tuberculosis in HIV-TB Co-infected Persons.
1. RAL vs. EFV for treatment of HIV-TB patients. The 48-week results of the ANRS 12 180 Reflate TB Trial were presented in abstract 853. The study compared the efficacy of efavirenz (EFV) against raltegravir (RAL) in HIV/TB co-infected patients receiving rifampin based (RIF) TB treatment. The study investigated the following treatment arms: RAL 400mg or 800mg twice daily or EFV 600mg daily. All regimens included TDF/3TC. The primary endpoint was the percentage of patients with HIV-RNA < 50 cpm at 48 weeks. 48 week success rates were RAL 400 BID, 76% (95% CI 65-88); RAL 800 BID, 63% (95% CI 49-76); and EFV, 67% (95% CI 54-80). There were no significant differences among the groups. These results would suggest RAL, at either the usual dose or the 800mg BID dose is comparable to EFV in HIV treatment regimens in HIV-TB co-infected individuals. Approximately 30% of persons in each treatment group developed ≥ grade 3 adverse events.
An important companion abstract is 539. This abstract showed the results of a drug-drug interaction study of RAL and rifampin in these HIV-TB co-infected patients. As above, patients were randomized to either 400 mg twice-daily RAL (the standard dose) or 800 mg twice-daily RAL, which is the increased dose recommended for management of the interaction with RIF. The PK results were highly variable but there was a trend toward lower concentrations of RAL (Cmax, C12, AUC) when 400mg BID was given with RIF. These effects were diminished in the group receiving RAL 800mg BID. The table below summarizes the concentrations seen in the two treatment groups.
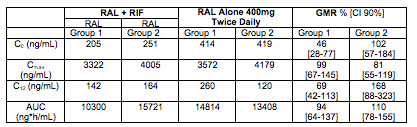
First, these data show rifampin substantially reduces RAL concentrations in HIV-TB patients, as was seen in healthy volunteers. Second, similar to what was seen in healthy volunteers, an increase in the RAL dose to 800 mg BID compensated for the decrease in AUC but not C12 (or trough concentration). The RAL C12 remained significantly reduced and was essentially the same on either the 400 or 800 mg BID dose (see Wenning L et al, Antimicrob Agents Chemother 2009;53:2852-56 for the healthy volunteer interaction data). Third, the RAL concentrations in these HIV-TB patients are much, much higher than those in healthy volunteers. For comparison, the mean RAL AUC and Cmax in the healthy volunteers at 400 mg BID without rifampin were 2050 ng*h/mL and 537 ng/mL, respectively. In the HIV-TB patients, at 400 mg BID without rifampin, the AUC and Cmax were (from Group 1 in above table) 14,800 ng*h/mL and 3572 ng/mL, respectively, - approximately a 7-fold increased exposure in the HIV-TB patients.
In this context now, the apparently equivalent efficacy of RAL 400 and 800 mg BID when given with rifampin makes some sense: the C12 concentrations with either dosing regimen were comparable (approximately 150 ng/mL, and were, for example, above/comparable to mean RAL trough concentrations of 112 ng/mL seen in the twice daily RAL arm that outperformed once daily RAL (see Eron J et al, Lancet Infect Dis 2011;11:907-15). The surprise to me then is what is the reason for the large difference in RAL concentrations between the HIV-TB patients and healthy volunteers. I haven't seen a difference of this magnitude, and there is a real need to understand if this is unique to the patients enrolled in the ANRS 12 180 Reflate TB Trial, or seen more broadly. Until we know, I would be very careful about extrapolating the results of this trial that RAL 400 and 800 mg BID equal efficacy when given with rifampin, too far.
2. PA-824 - a new investigational anti-TB drug. Kelly Dooley presented data from ACTG A5306 describing the safety and PK of PA-824, an investigational anti-TB drug, when given with EFV in healthy subjects (abstract 188LB). Within the 16 subjects who completed the interaction study with once daily PA-824 200mg and EFV 600mg, it was found that EFV lowered PA-824 AUC and trough concentrations by 35% and 46%. The relationship between PA-824 concentrations and TB response and appropriate dose of PA-824 needs to be determined to know whether this interaction is clinically significant. However, given the magnitude of this interaction, my suspicion is this interaction will require some management strategy, such as an increased dose of PA-824.
IV. New Drugs for Hepatitis C
A real highlight of CROI this year was the progress made in the discovery, development and clinical pharmacology of drugs to treat hepatitis C (HCV). CROI: HCV at CROI (new HCV drugs)- (03/17/13)
1. Interactions among current HCV and HIV drugs. One of the most innovative presentations was abstract 34, which investigated ribavirin (RBV) concentrations in cells and plasma in subjects receiving telaprevir (TVR). They observed increased RBV concentrations in the subjects receiving TVR: RBV concentrations increased 1.54-fold in subjects receiving TVR, as compared with those who did not receive TVR. They suggest this interaction may be a cause of the increased rates of anemia seen with the combination of RBV and TVR. In related work, poster 538 examined intracellular RBV concentrations in HCV+ (but HIV uninfected) individuals who were also treated with abacavir (ABC). The researchers did not observe altered RBV plasma or intracellular concentrations in subjects receiving ABC, indicating the two medications can be safely combined in HIV/HCV dual-infected individuals. Poster 537 investigated a potential interaction between BOC and rilpivirine (RPV), and found that BOC increased the AUC of RPV by 39% when administered simultaneously. The increase in RPV concentrations is not likely to be clinically significant, and the two drugs can be administered simultaneously without the need for a dose adjustment, with an important caveat: as long as there are not other drugs in the regimen that also increase RPV concentrations. (http://www.natap.org/2013/CROI/croi_141.htm)
2. Faldaprevir and ARV Interactions. Presentation 40LB reported on a phase 3 trial assessing the rate of HCV response in HCV/HIV co-infected individuals receiving faldaprevir (FDV), pegylated interferon, and RBV. They observed that subjects receiving this combination had high virologic response at weeks 4 and 12; approximately 80% met criteria for early treatment success at week 12. The safety profile was similar to other utilized treatments. Presentation 35 investigated PK interactions between FDV and a number of currently used ARV drugs. FDV concentrations were increased by DRV/RTV, and decreased by TFV and EFV. To manage these interactions in the trial discussed above (40LB), the dose of FDV was 120 mg once daily if given with DRV/RTV, and was 240 mg once daily if given with EFV. See also these NATAP reports:
http://www.natap.org/2013/CROI/croi_03.htm
http://www.natap.org/2013/CROI/croi_02.htm
3. Sofosbuvir. Sofosbuvir (SOF) has to be one of the more exciting novel anti-HCV drugs in development. Presentation 157LB described a trial utilizing SOF and RBV in subjects categorized as difficult to treat (high HCV viral load, high BMI, black race, and advanced liver fibrosis). In these subjects they observed a marked decrease in HCV RNA (approximately a 4 log decline in viral RNA in SOF + RBV group) as compared with other treatment approaches in these difficult to treat subjects. Preliminary results from the ELECTRON trial were given in 41LB, which investigated therapy with SOF, RBV, and ledipasvir (LDV) in both treatment naïve and experienced individuals (null responders). At week 12, sustained virologic response rates (SVR12) were as follows. Treatment-naïve: SOF+RBV, 84%; SOF+LDV+RBV, 100%. Null responders: SOF+RBV, 10%; SOF+LDV+RBV, 100%. Adverse effects were mainly mild adverse effects and consistent with those associated with RBV. A phase 3 clinical trial is now underway evaluating a fixed dose tablet of SOF+LDV (400mg+90mg) given once daily with or without RBV in treatment experienced HCV genotype 1 persons (ION-2; NCT01768286).
Two abstracts presented at the AASLD meeting in November 2012 on the drug interaction profile of SOF are worth mentioning. In studies in healthy volunteers, SOF had no clinically significant interactions with cyclosporine and tacrolimus, or with the ARVs TDF, FTC, EFV, RPV, or DRV/RTV (abstracts 1869 and 1877, Hepatology, 56, 4 suppl, October 2012). The AUC and Cmax of RAL were decreased 27% and 43% when given with SOF; coadministration may be possible but some clinical validation is necessary before I would conclude this interaction is not clinically significant. Nonetheless, if SOF is ultimately shown to be safe and effective for treatment of HCV infection, its drug-drug interaction profile will represent a significant improvement over existing agents.
CROI: High Efficacy of Sofosbuvir in Combination with Weight Based Ribavirin for 24 weeks in Difficult to Treat HCV Infected Genotype-1 Patients - (03/06/13)
CROI: ELECTRON: 100% SVR Rate for Once-Daily Sofosbuvir Plus Ledipasvir Plus Ribavirin Given for 12 Weeks in Treatment-Naïve and Previously Treated Patients With HCV GT 1 - (03/04/13)
V. Compartments, Reservoirs and Cure Research.
It was exciting to me to see the vibrancy of the compartments, reservoir and cure research agenda at CROI 2013. As should be expected, there were some disappointments, but some progress forward.
1. CSF-targeted ARV therapy. Abstract #20 presented data from a randomized control trial of CNS targeted therapy to see if a CSF targeted regimen versus non-CSF targeted regimen improved neurocognitive performance in persons with HIV-associated neurocognitive disorders (HAND). This trial was terminated early by the Data Safety and Monitoring Board (DSMB) because of futility (a low likelihood of detecting a difference in the primary outcome) and slow accrual. At termination there were 59 participants. An intention to treat analysis was performed on 49 subjects: there was no difference in neurocognitive performance with the CSF targeted ARV regimen. Though this trial of CSF targeted therapy didn't show a benefit, I do remain enthusiastic about the general idea of reservoir-targeted therapy as part of the cure agenda - the challenge is whether we can actually achieve effective concentrations of ARVs in the desired reservoir(s). (http://www.natap.org/2013/CROI/croi_144.htm)
2. Vorinostat. Histone deacetylase (HDAC) inhibitors, and vorinostat in particular received lots of attention last year at CROI 2012. They did at CROI 2013, but the news wasn't as positive. HDACs are being investigated as part of a strategy to eliminate the latent reservoir of HIV. Posters including 371, 378, and 379 investigated vorinostat, or other means to attempt to activate latent virus to allow it to be eliminated by ART. To date, significant reactivation of latent virus has not been achieved. Abstract 50LB reported the results of a multiple dose trial of vorinostat in HIV-infected persons receiving ARV therapy. After 14 days of therapy, there was no change in HIV DNA. These negative results don't kill HDACs as a class of drugs, or research on induction and clearance as a strategy for the treatment of latent HIV and viral eradication. There are more potent HDACs and other approaches worth pursuing.
3. Intensification - with maraviroc and with raltegravir. Two abstracts focused on intensification of therapy in virally suppressed individuals. One study from the University of Hawaii (abstract #403), added maraviroc (MVC) for 24 weeks to virally suppressed patients (RNA < 50 copies/mL for > 6 months) currently on ART. Neuropsychological performance improved and lower rates of immune system activation as measured by frequency of activated monocytes, lower soluble CD14 (an immune activation marker) plasma levels, and lower percentages of activated CD8+ T-cells versus pre-MVC treatment measurements were achieved.
Abstract #42 was an intensification study of RAL in ART suppressed HIV+ individuals with high CD4+ T-cell counts (≥350 cells/mm3). In this randomized, placebo controlled, double-blind study, subjects were randomized to receive intensification with RAL 400mg twice daily or placebo in addition to their current ART. The authors found that while RAL did not have a significant effect on plasma HIV RNA levels (single copy assay) it did have a significant effect on levels of 2-LTR circles. Subjects in the RAL group had a significantly higher level of 2-LTR circles at every week analyzed, beginning with week one of RAL intensification. These results suggest that RAL intensification has a modest effect at lowering residual levels of viral replication.
4. A benefit to ART in asymptomatic HIV controllers? Hiroyu Hatano and colleagues presented very provocative data from study of HIV controllers (abstract 75LB) treated with prospective ART. HIV controllers are individuals who are able to maintain low levels of plasma viremia without the need for ART. ART consisting of RAL/TDF/FTC for 24 weeks was given to 16 controllers. Significant decreases in both plasma viremia (as measured by single copy assay) and markers of immune activation in both blood and GALT tissue were observed. These data may have implications for chronic inflammation in HIV controllers and suggests ART should be considered in this population.
A closing comment on the cure agenda. I am excited about the prospects for new drug discovery and strategies such as induction and clearance. However, I think there needs to be an increased focus on developing ARV regimens that are fully suppressive in all compartments and reservoirs, as I think full suppression is a necessary prerequisite to the success of any eradication strategy.
|
| |
|
 |
 |
|
|