 |
 |
 |
| |
New Data from AbbVie's Phase IIb Aviator Trial Demonstrate High Sustained Viral Response Rates Across Multiple Patient Types with HCV Genotype 1 - press release
|
| |
| |
· 96 percent of treatment-naïve patients and 93 percent of prior null responders treated with AbbVie's investigational IFN-free, triple-DAA combination therapy achieve SVR24
· Additional analyses looks at response rates in patients with differing baseline factors including gender, viral load and fibrosis stage
AMSTERDAM, Apr. 23, 2013 (NYSE: ABBV) - Results from "Aviator," AbbVie's phase IIb clinical trial of its investigational direct-acting antivirals (DAAs) for the treatment of hepatitis C virus (HCV) infection, continue to demonstrate high sustained viral response (SVR) rates against genotype 1 HCV, across patient types. Data show greater than 90 percent SVR were achieved in patients new to treatment and in patients who had previously failed treatment with pegylated interferon and ribavirin (null responders).
In addition, similar high SVR rates observed after 12 and 24 weeks of treatment in the Phase IIb trial reinforce the adequacy of the 12-week treatment duration for the investigational interferon-free, triple DAA combination. The triple-DAA combination is currently being studied in Phase III clinical trials. Results will be featured in the official press conference at the 2013 International Liver Congress® (ILC) in Amsterdam on Wednesday, April 24 at 11:00 CEST and presented on Thursday, April 25.
"These new results from the Aviator study further demonstrate that this investigational all-oral therapy combination can achieve high sustained viral response after 12 weeks of treatment," said Kris Kowdley, M.D., Director of the Liver Center of Excellence and Director of Research in the Digestive Disease Institute at Virginia Mason Medical, and Clinical Professor of Medicine at the University of Washington in Seattle. "The consistency of high sustained viral response rates that we have seen in clinical trials across populations is encouraging, especially given the proportion of patients with these characteristics who have failed with interferon plus ribavirin treatment."
"AbbVie's clinical development program aims to improve virologic cure rates, including in patients who have historically been harder to treat with current therapies, such as prior null responders. While further studies are required to confirm these findings, we remain encouraged by the high viral response rates and the safety profile we have seen in the Aviator study," said Barry Bernstein, divisional vice president, infectious disease development, AbbVie. "Our Phase III trials are progressing well and we remain focused on bringing an interferon-free treatment option to patients with HCV genotype 1 infection."
About Study M11-652 (Aviator)
The objective of this phase 2b study was to assess the safety, and efficacy of ABT-450/r (dosed 100/100 to 200/100mg once daily), ABT-267 (25mg once daily), ABT-333 (400mg twice daily) and ribavirin in non-cirrhotic treatment-naïve patients and prior peg-interferon/ribavirin null responders administered for 8, 12 or 24 weeks. Enrollment was open to GT1-infected patients regardless of IL28B host genotype and ribavirin dosing was weight-based.
A summary of key data from the trial is below:
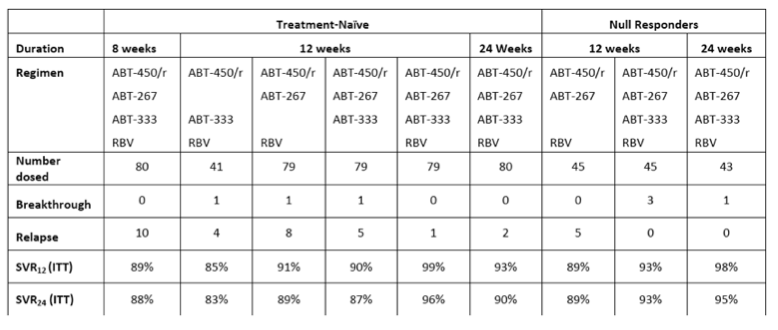
For the 12-week triple-DAA regimen with ribavirin being studied in Phase 3 trials:
· 99% of treatment-naïve patients achieved SVR12, 96% achieved SVR24 in this intent-to-treat analysis
· 93% of prior null responders achieved SVR12 and SVR24
· The single relapse with this regimen occurred at post-treatment week two
With the triple-DAA plus ribavirin regimen, comparable SVR24 response rates were also seen in treatment naïve patients and null responder patients across HCV subtype, IL28B genotype and baseline HCV-RNA levels and severity of fibrosis.
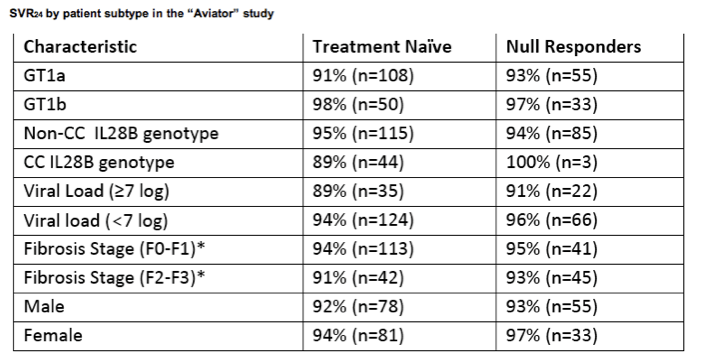
*The fibrosis analysis was post-hoc based on biopsy or non-invasive testing at screening.
The safety profile seen in this study is consistent with the initial presentation of results in November 2012. Of the 247 patients included in this analysis, four patients (1.6 percent) discontinued the study because of drug-related adverse events. Serious adverse events were noted in 4 patients (1.6 percent), with one (arthralgia) considered possibly drug-related. Other events reported in more than 10 percent of patients included headache, fatigue, nausea, insomnia, and diarrhea. Grade 3-4 laboratory abnormalities in total bilirubin (six patients) and ALT (one patient) were noted; all resolved with continued dosing.
About Study M11-652 (Aviator)
The objective of this phase 2b study was to assess the safety, and efficacy of ABT-450/r (dosed 100/100 to 200/100mg once daily), ABT-267 (25mg once daily), ABT-333 (400mg twice daily) and ribavirin in non-cirrhotic treatment-naïve patients and prior peg-interferon/ribavirin null responders administered for 8, 12 or 24 weeks. Enrollment was open to GT1-infected patients regardless of IL28B host genotype and ribavirin dosing was weight-based.
About the Hepatitis C Virus
Across the world, about 160 million people are chronically infected with hepatitis C.1 Hepatitis C is an inflammation of the liver caused by an infection with the hepatitis C virus (HCV).2 HCV is transmitted when an infected person's blood enters the bloodstream of another person.3
For the hepatitis C virus, there are six major HCV genotypes (GT1-6).4 Presently, there is no vaccine for the hepatitis C virus (HCV) infection.3 Decision to treat is dependent on a number of factors such as the amount of liver damage present, other conditions the patient may have, amount of virus in the body, and viral genotype.4 If treatment is needed, a hepatitis C infection is typically treated with a combination of antivirals.3
About AbbVie's HCV Development Programs
AbbVie's HCV portfolio includes investigational medicines with three different mechanisms of action, including protease (ABT-450/r), polymerase (ABT-333) and NS5A (ABT-267) inhibitors, currently being studied in clinical trials. ABT-450 is being developed with low dose ritonavir which enhances the pharmacokinetic properties of ABT-450. The use of ritonavir 100mg with ABT-450 for the treatment of HCV is investigational.
ABT-450 was discovered during the course of a collaboration between AbbVie and Enanta Pharmaceuticals for HCV protease inhibitors and regimens that include protease inhibitors. ABT-450 is being developed by AbbVie for use in combination with AbbVie's other investigational medicines for the treatment of HCV. AbbVie is well-positioned to explore combinations and co-formulations of these medicines.
Ritonavir Use in the Treatment of HIV
Ritonavir is in a class of medicines called the HIV protease inhibitors. Ritonavir is used in combination with other anti-HIV medicines to treat people with human immunodeficiency virus (HIV) infection. Ritonavir is for adults and for children greater than 1 month in age and older.
Ritonavir does not cure HIV infection or AIDS and does not reduce the risk of passing HIV to others. People taking ritonavir may still get opportunistic infections or other conditions that happen with HIV infection. Some of these conditions are pneumonia, herpes virus infections, and Mycobacterium avium complex (MAC) infections.
Ritonavir Safety in Treatment of HIV
Patients should not take ritonavir with certain medicines, as these can cause serious or life-threatening problems such as irregular heartbeat, breathing difficulties, or excessive sleepiness. Patients should not take ritonavir if they have had a serious allergic reaction to any of its ingredients. Some patients taking ritonavir may develop liver and pancreas problems, which can cause death.
Patients may develop large increases in triglycerides and cholesterol, diabetes, high blood sugar, changes in body fat, increased bleeding in people with hemophilia, allergic reactions, and/or changes in heart rhythm. Patients may develop signs and symptoms of infections that they already have after starting anti-HIV medicines.
For more information, please see Important Safety Information and Full Prescribing Information.
About AbbVie
AbbVie is a global, research-based biopharmaceutical company formed in 2013 following separation from Abbott. With its 125-year history, the company's mission is to use its expertise, dedicated people and unique approach to innovation to develop and market advanced therapies that address some of the world's most complex and serious diseases. In 2013, AbbVie employs approximately 21,000 people worldwide and markets medicines in more than 170 countries. For further information on the company and its people, portfolio and commitments, please visit www.abbvie.com. Follow @abbvie on Twitter or view careers on our Facebook or LinkedIn page.

|
| |
|
 |
 |
|
|