 |
 |
 |
| |
Phase 3 Randomized Controlled Trial of All-Oral Treatment With Sofosbuvir + Ribavirin for 12 Weeks Compared to 24 Weeks of PEG + Ribavirin in Treatment-Naïve GT 2/3 HCV-Infected Patients (FISSION)
|
| |
| |
Reported by Jules Levin
International Liver Congress 2013, Amsterdam April 25
Edward Gane1, Eric Lawitz2, Maribel Rodriguez-Torres3, Stuart Gordon4, Hadas Dvory-Sobol5, Sarah Arterburn5, John McNally5, Diana M. Brainard5, William T. Symonds5, John G. McHutchison5, Aasim Sheikh6, Alessandra Mangia7
1Auckland City Hospital, Auckland, New Zealand; 2Texas Liver Institute, San Antonio, TX, USA; 3Fundacion de Investigacion, San Juan, Puerto Rico; 4Henry Ford Health Systems, Detroit, MI, USA; 5Gilead Sciences, Inc., Foster City, CA, USA; 6GI Specialists of Georgia, Marietta, GA, USA;
7"Casa Sollievo della Sofferenza" Hospital, San Giovanni Rotondo, Italy
ELECTRON: 100% SVR Rate for Once-Daily Sofosbuvir Plus Ledipasvir Plus Ribavirin Given for 12 Weeks in Treatment-Naïve and Previously Treated Patients With HCV GT 1........http://www.natap.org/2013/CROI/croi_07.htm
NEJM - Sofosbuvir for Hepatitis C Genotype 2 or 3 in Patients without Treatment Options - (04/24/13)
The Lancet Infectious Diseases- Sofosbuvir in combination with peginterferon alfa-2a and ribavirin for non-cirrhotic, treatment-naive patients with genotypes 1, 2, and 3 hepatitis C infection: a randomised, double-blind, phase 2 trial - (04/24/13)
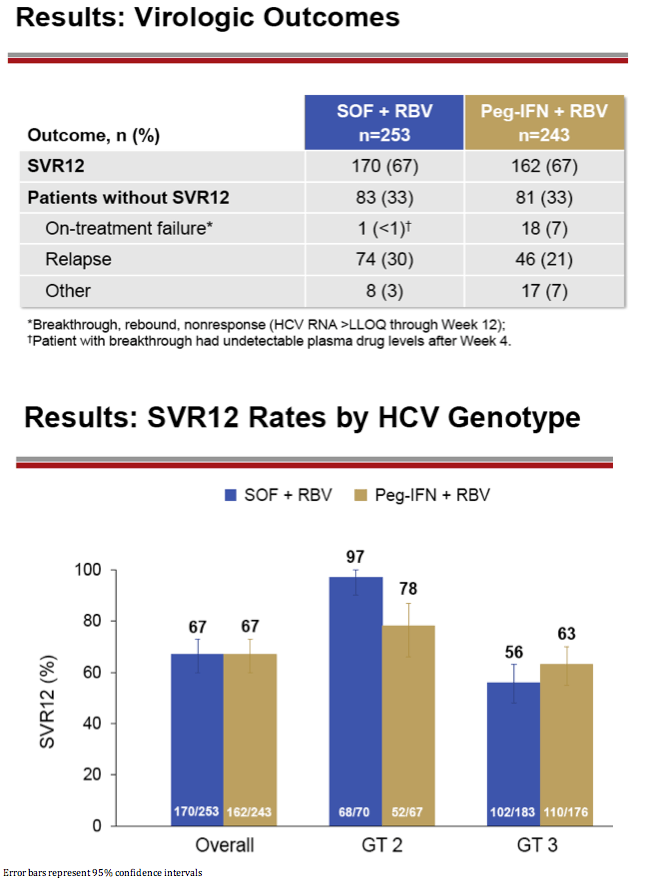
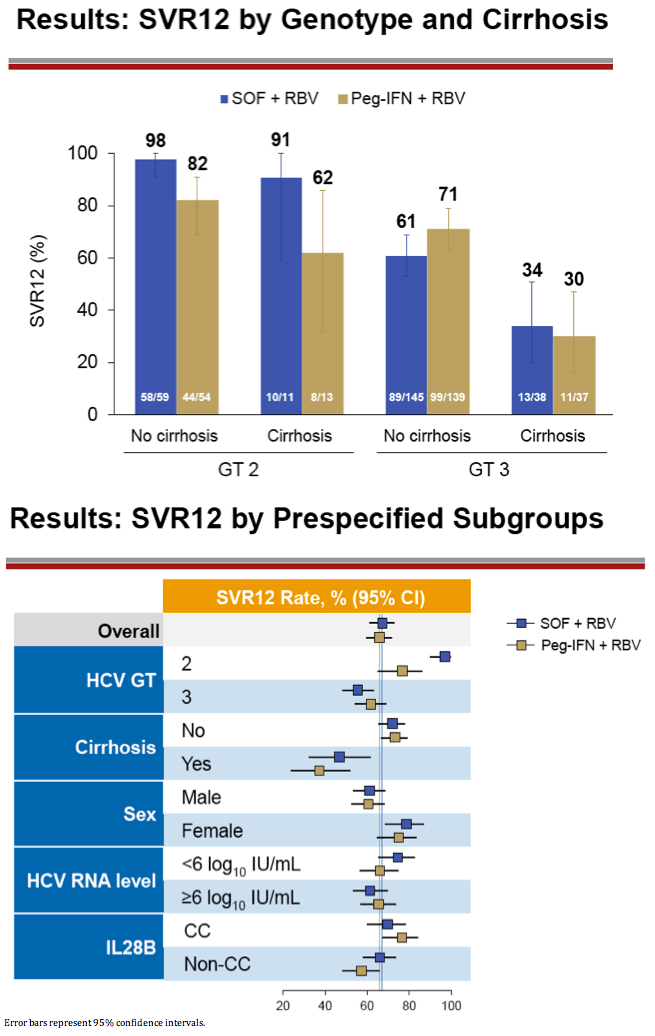
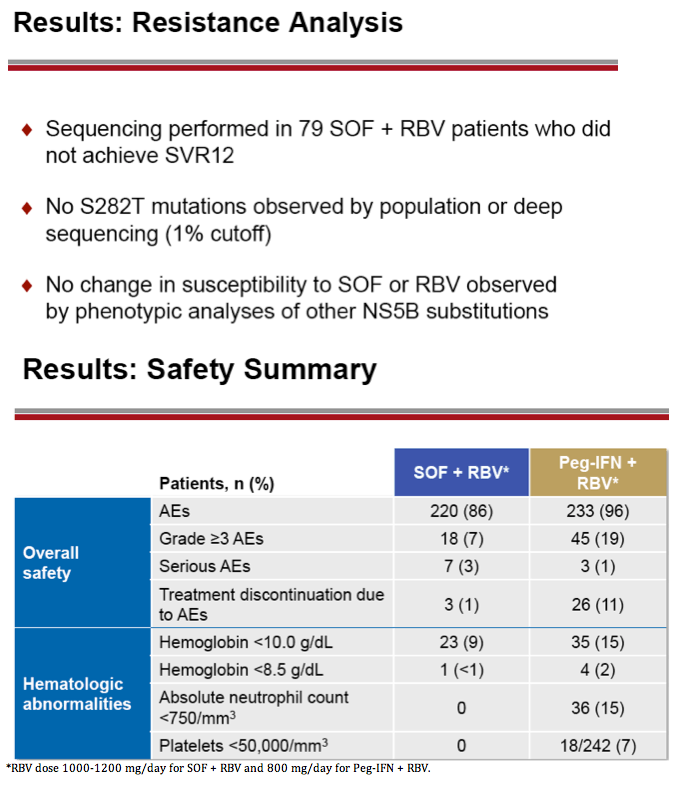
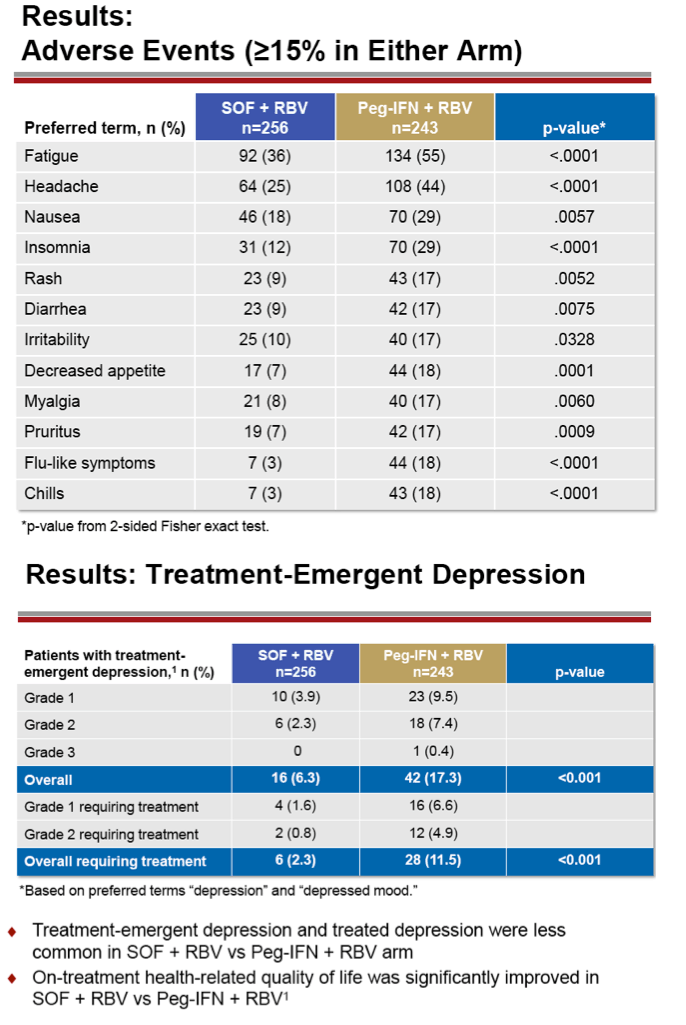
|
| |
|
 |
 |
|
|