| |
Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection
|
| |
| |
Download the PDF here
Hepatology May 2013
Tetsuya Hosaka,1 Fumitaka Suzuki,1 Masahiro Kobayashi,1 Yuya Seko,1 Yusuke Kawamura,1 Hitomi Sezaki,1 Norio Akuta,1 Yoshiyuki Suzuki,1 Satoshi Saitoh,1 Yasuji Arase,1 Kenji Ikeda,1 Mariko Kobayashi,2 and Hiromitsu Kumada
Pre-existing cirrhosis is the key, the 2nd leading, risk factor for developing HCC
Abstract
Chronic hepatitis B virus (HBV) infection leads to cirrhosis and hepatocellular carcinoma (HCC). Antiviral agents are thought to reduce HCC development, but agents such as lamivudine (LAM) have a high rate of drug resistance. We compared the incidence of HCC in 472 entecavir (ETV)-treated patients and 1,143 nontreated HBV patients (control group). Propensity score matching eliminated the baseline differences, resulting in a sample size of 316 patients per cohort. The drug mutation resistance was 0.8% (4/472) in the ETV group. The cumulative HCC incidence rates at 5 years were 3.7% and 13.7% for the ETV and control groups, respectively (P < 0.001). Cox proportional hazard regression analysis, adjusted for a number of known HCC risk factors, showed that patients in the ETV group were less likely to develop HCC than those in the control group (hazard ratio: 0.37; 95% confidence interval: 0.15-0.91; P = 0.030). Both cohorts were applied in three previously reported risk scales and risk scores were generated based on age, gender, cirrhosis status, levels of alanine aminotransferase, hepatitis B e antigen, baseline HBV DNA, albumin, and bilirubin. The greatest HCC risk reduction occurred in high-risk patients who scored higher on respective risk scales. In sub analyses, we compared treatment effect between nucleos(t)ide analogs, which included matched LAM-treated patients without rescue therapy (n = 182). We found HCC suppression effect greater in ETV-treated (P < 0.001) than nonrescued LAM-treated (P = 0.019) cirrhosis patients when they were compared with the control group. Conclusion: Long-term ETV treatment may reduce the incidence of HCC in HBV-infected patients. The treatment effect was greater in patients at higher risk of HCC. (HEPATOLOGY 2013)
More than 2 billion people worldwide have been exposed to hepatitis B virus (HBV) and about 350 million people are chronically infected, the majority of whom are in Asia (75%). The prevalence of HBV in Japan is 0.8%, which is lower than other Asian countries such as Taiwan (>10%) and China.1-3 As chronic HBV infection leads to cirrhosis and hepatocellular carcinoma (HCC), published studies have shown that up to 25% of chronically infected patients eventually die of liver cirrhosis or HCC.4
A large-scale longitudinal epidemiologic study has shown that a patient's baseline HBV DNA level is an independent predictor for the development of HCC.5 Studies have begun to show that treatment to decrease HBV DNA reduces the risk of HCC development in HBV patients with cirrhosis or advanced fibrosis or in chronic HBV patients.6, 7
Within the past 10 years, new antiviral therapies, including nucleos(t)ide analogs (NAs), have been approved and were successful in suppressing circulating serum viral loads. Studies that have examined the relationship between NA therapy and HCC almost exclusively used older drugs such as lamivudine and/or adefovir. Although results of long-term studies showed the importance of antiviral suppression, HCC risk among patients treated by newer NAs remains inconclusive. Entecavir (ETV) is a relatively new antiviral NA that has proved effective in suppressing HBV DNA replications with minimal drug resistance.8, 9 In this study we examined whether long-term ETV treatment would reduce HCC risk in HBV-infected patients when compared with NA-naïve patients.
Abbreviations
ALT, alanine aminotransferase; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; ETV, entecavir; HBeAg, hepatitis B e antigen; HBV DNA, hepatitis B virus deoxyribonucleic acid; HR, hazard ratio; NA, nucleos(t)ide analogs; PS, propensity score; ROC, receiver operating characteristic curve.
Patients and Methods
Patients and Design.
From 2004 to 2010, we consecutively recruited 510 patients treated with 0.5 mg ETV (ETV group); the ETV group was compared with a retrospective cohort of 2,332 NA-naïve, HBV-infected patients (control group).
These patients were chronically monoinfected with HBV and were confirmed as hepatitis B s antigen (HBsAg)-positive for at least 6 months. As a general rule, ETV was initiated in a patient who had both abnormal alanine aminotransferase (ALT) levels (defined as ALT ≥45) and elevated HBV DNA levels of ≥4 log copies/mL. A patient with advanced fibrosis would be treated with ETV if the ALT level was normal; however, a patient without fibrosis or with a normal HBV DNA/ALT level would not be treated with ETV. Among the treated patients, 38 were excluded from the ETV group either because their follow-up period was less than 1 year (n = 28) or because the clinical data or serum samples were incomplete (n = 10). The remaining 472 ETV-treated patients were included in the analysis (Fig. 1). No patient in the ETV group received other NAs before ETV treatment.
The control group patients were recruited from 1973 to 1999. These patients were NA-naïve at baseline, as no NA therapy had yet been approved. Patients were excluded from the control group if (1) their follow-up duration was less than 1 year (n = 262); (2) corticosteroid withdrawal therapy (n = 120), IFN treatment (n = 305) or NA treatment (n = 273) was initiated during follow-up; (3) clinical data or serum samples were incomplete (n = 153); or (4) patients were found to be positive for anti-hepatitis C virus antibodies (HCV-Ab) (n = 76). The remaining 1,143 patients served as the control population (Fig. 1).
We also made subanalyses to examine the difference of HCC suppression effect between NAs. To make this comparison, we recruited a cohort of 949 consecutive patients from our hospital who were treated with lamivudine (LAM) (September 1995 to September 2007). LAM-treated patients who met the same inclusion criteria as the ETV group, who had no rescue therapy (LAM group, n = 492), were used in the comparison.
We received informed consent from each patient at their entry into the study. Informed consent for the clinical data collection and storage of serum samples were obtained from each patient in the historical control group. The study protocol was in accordance with the ethical guidelines of the Declaration of Helsinki and approved by the Toranomon Hospital Ethics Committee.
Clinical Data Collection and Follow-up.
All ETV-treated and untreated patients were followed at 1- to 3-month intervals, during which biochemical and HBV virological markers, blood counts, tumor markers (e.g., alpha-fetoprotein and des-γ-carboxylprothrombin), and cirrhosis and HCC status were monitored. Viral response in the ETV group was defined as a reduction in HBV DNA levels to below 400 copies/mL. Cirrhosis was determined by laparoscopy, liver biopsy, imaging modalities, or portal hypertension. HCC was diagnosed predominantly via imaging, including dynamic computed tomography, magnetic resonance imaging, and/or digital subtraction angiography. When the hepatic nodule did not show typical imaging features, diagnosis was confirmed by fine-needle aspiration biopsy followed by histological examination. Patients were followed until any confirmed HCC diagnosis 1 year after the start of observation (primary outcome) or until the last visit before December 2011. All patients also underwent ultrasonography or helical dynamic computed tomography every 3 to 6 months (cirrhosis patients) or every 6 to 12 months (noncirrhosis patients).
HBV Infection Markers.
HBV DNA levels were quantified using the COBAS Amplicor HBV Monitor Test (Roche Diagnostics, Tokyo, Japan), which has a dynamic range of 2.6 to 7.6 log copies/mL, or COBAS TaqMan HBV Test v2.0 (Roche Diagnostics) which has a dynamic range of over 2.1 to 9.0 log copies/mL. HBV DNA of the control group was measured from their stored frozen serum (-80°C) using COBAS TaqMan HBV v.2.0 once at the start of observation. Previous measurements were taken using the old DNA polymerase assay in the control group and thus were not used for comparisons. For the ETV group, drug-resistant mutations were determined from a nested polymerase chain reaction, using a primer specific at the polymerase region in patients who had an HBV DNA relapse of ≥1 log copies from nadir. Hepatitis B e antigen; (HBeAg) was determined by enzyme-linked immunosorbent assay with a commercial kit (HBeAg EIA; Institute of Immunology, Tokyo, Japan). A commercial kit (HBV Genotype EIA; Institute of Immunology) was used to serologically determine HBV genotypes using the combination of epitopes expressed on the pre-S2 region product, which is specific for each of the eight major genotypes (A to H).
HCC Incidence by Risk Scores.
To examine HCC incidence by risk scores, we applied published HCC risk scales, which are based on the natural course of HCC among HBV-positive patients, to our cohorts. We first searched Medline/PubMed using "hepatitis B," "cancer," and "risk score" as keywords and found four publications in English that used risk-score estimations.10-13 One article was rejected because we were unable to compute the risk scores with our variables, and therefore we used only the scales indicated by the remaining three publications to generate the risk scores.13 The risk scales were based on parameters such as age, gender, cirrhosis, levels of ALT, HBeAg, baseline HBV DNA, albumin, and bilirubin. The original risk score formula and the risk score distributions for our two cohorts derived from these formulas are shown in Supporting Table 1. The risk score cutoff points were determined from the following original articles. In Yang et al.'s article,10 the risk score was derived from 17-point categories. When we applied the scores to our control group, we found that the 12-point scale was at best in detecting a difference in HCC incidence. With that, we examined the HCC suppression treatment effect by dividing the patients into equal halves with 12 points as the cutoff. Yuen et al.11 divided their cohort in half and found risk scores of 82 as the optimal cutoff point. We also applied the same cutoff point to our cohorts. Wong et al.12 used their risk scores to categorize their cohort into low-risk, medium-risk, and high-risk groups with respective cutoff points at <4, 4-19, ≥20. We also applied the same cutoff points to our cohorts to examine the treatment effect. Cumulative HCC incidence rates were compared by these risk scores between the ETV and control groups.
Statistical Analysis.
Categorical data were compared using chi-square or Fisher's exact tests. Continuous variables with normal distributions were compared using Student's t test, and those without normal distributions were compared using the Mann-Whitney U test. Cumulative HCC incidence rates were analyzed using the Kaplan-Meier method; patients followed beyond 5 years were censored to better compare the two cohorts because the ETV group had a shorter follow-up period when compared with the historical control group. We compared the cumulative incidence of HCC using the log-rank test, and Cox proportional hazard regression analysis, which was used to assess the variables that were significantly associated with the development of HCC. Deaths before HCC development were censored. Significance was defined as P < 0.05 for all two-tailed tests.
We used the propensity score (PS) matching method to reduce significant differences in demographics between the ETV and control groups.14, 15 Using multiple logistic regression analysis, a PS was estimated for all patients treated with ETV.14 Variables used in the model included age, sex, presence of cirrhosis, HBeAg, HBV DNA< aspartate aminotransferase (AST), ALT, γ-glutamyl transpeptidase; (γ-GTP), bilirubin, albumin, and platelet counts. We performed caliper matching on the PS (nearest available matching). Pairs (ETV and the control group) on the PS logit were matched to within a range of 0.2 standard deviation (SD).16, 17 The PS logit distributions for each cohort showing the overlaps and SD ranges are shown in Supporting Fig. 1. The balance of covariates was measured by their standardized differences. A difference >10% of the absolute value was considered significantly imbalanced.17 The cohorts were divided into five PS quintiles (Supporting Table 2). We also made subanalyses to examine the difference of HCC suppression effect between NAs by comparing the HCC incidence between propensity score matched ETV- and lamivudine (LAM)-treated patients without a rescue therapy. The LAM-treated patients were derived from consecutive sampling at our institution and were PS matched with ETV group according to the same method described above. Interaction of the subgroups by preexisting cirrhosis or risk scores and ETV treatment were evaluated. P < 0.10 was considered statistically significant. Data analysis was performed using IBM SPSS v. 19.0 software (Armonk, NY) and R software v. 2.13 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org).
Results
Patient Characteristics.
The patient characteristics at the baseline, before PS matching are shown in Table 1. The ETV group was followed for an average of 3.2 years (1,561 person-years), whereas the control group was followed for an average of 9.5 years (12,381 person-years). Before matching, patients in the ETV group and the control group differed significantly in age, gender, genotype, baseline HBV DNA level, and other clinical data. In the ETV group, 421 patients (89%) had HBV DNA (<400 copies/mL) at year 1. Not all patients in the control group were tested for HBV DNA level during follow-up. The drug mutation resistance was 0.8% (4/472). The four patients who had drug mutation did not develop HCC. During follow-up, 12 patients (2.54%) in the ETV group and 144 patients (12.60%) in the control group developed HCC. The incidence rates of HCC for the ETV and the control groups were 76/10,000 patient-years and 116/10,000 patient-years, respectively. During this period, 21 patients in the control group developed liver cirrhosis while no patient developed liver cirrhosis in the ETV group. During the same observation period, there were four deaths in the ETV group and 10 deaths in the control group. We took competing risk into account18, 19 and compared incidence of non-HCC deaths between the cohorts and the results were not different. However, because there were only four patients in the non-HCC deaths in the ETV group (two patients in the PS matched cohort) and 10 patients in the control group (six patients in the PS matched cohort), we considered that it was not meaningful to apply competing risk analysis in our cohorts.
Factors Associated with HCC and Effect of ETV Treatment on HCC Development.
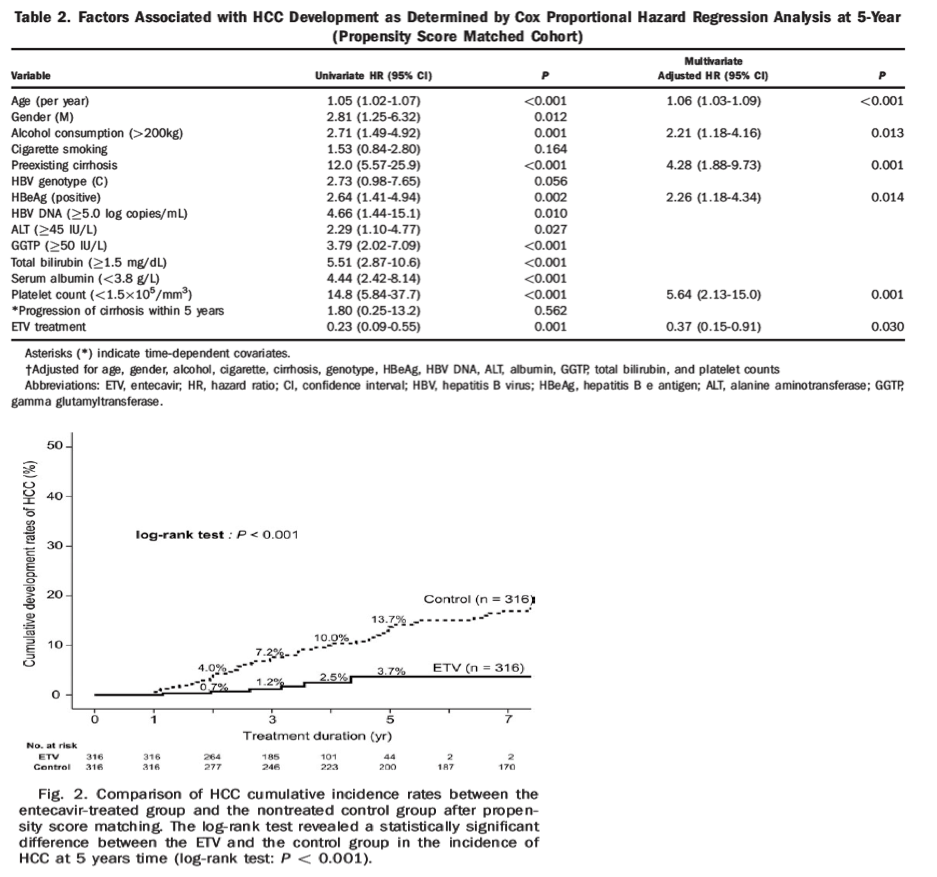
To allow a common ground for comparison between the two cohorts, we used PS matching with selected key characteristics and compared the two groups within the same time period of 5 years. The PS matching process resulted in a matched sample size that consisted of 316 patients in each group (Table 1). The PS matching reduced the significant variability of the two cohorts. While five (42%) of the 12 covariates varied by >10% before matching, all covariates differed by <10% of the absolute value after matching (Supporting Fig. 2). In the PS score matched cohort, 10 out of the 231 noncirrhosis patients progressed to liver cirrhosis within the 5 years of observation. The cumulative incidence rates of HCC in the matched ETV groups were 0.7% at year 2, 1.2% at year 3, 2.5% at year 4, and 3.7% at year 5. The cumulative incidence rates of HCC in the matched control group were 4.0% at year 2, 7.2% at year 3, 10.0% at year 4, and 13.7% at year 5. Log-rank test revealed a statistically significant difference between the incidence of HCC in the ETV group and the control group over time (P < 0.001) (Fig. 2). We then used Cox proportional regression analysis to estimate the effects of ETV treatment on HCC risk. Factors that were associated with HCC at year 5 in the propensity score matched cohort were age, gender, alcohol consumption (>200 kg), the presence of cirrhosis, HBeAg positivity, baseline viral load, ALT, γ-GTP, total bilirubin, serum albumin, and platelet counts (Table 2). For ETV treatment effect, we estimated the hazard ratio of HCC development, adjusting for multiple baseline variables (age, gender, alcohol consumption, smoking, preexisting cirrhosis, HBeAg, HBV DNA, ALT, albumin, γ-GTP, total bilirubin, and platelet count) in the propensity matched cohort. Progression of cirrhosis within 5 years was used as a time-dependent covariate in the proportional hazard regression but it did not show a statistically significant hazard to HCC development.
Subanalyses Showing HCC Suppression Effect Between ETV and LAM.
PS matching of the LAM-treated patients without rescue therapy (n = 492) with ETV-treated patients resulted in a matched cohort of 182 patients (Supporting Table 3). The rate of nonrescued LAM-treated group having undetectable HBV DNA at 1 year after treatment was lower when compared with the ETV-treated group. The LAM-treated group also had a higher drug-resistant mutation rate. Comparisons of HCC incidence among the ETV-treated group, nonrescued LAM-treated group, and control showed that the HCC suppression effect was greater in ETV-treated (P < 0.001) than nonrescued LAM-treated (P = 0.019) when compared with the control group (Fig. 3). The difference of effect between ETV and LAM was also significant (P = 0.043). The treatment effect was seen in cirrhosis patients but not in noncirrhosis patients. The result showed ETV's superiority to LAM in suppressing HCC.
Effect of ETV on the Reduction of HCC Development by Preexisting Cirrhosis and Risk Scores.
To further examine the ETV treatment effect, we compared the ETV and the control groups by preexisting cirrhosis and published risk scores. Viral response rates (HBV DNA < 400 copies/mL) of 1-year post-ETV treatment was 87% in the noncirrhosis patients and 91% in the cirrhosis patients (LC). ALT normalization was 94% and 90% in the chronic hepatitis and cirrhosis patients, respectively. The treatment effect was not inferior by cirrhosis status. Among those who developed HCC, 97 out of 144 patients in the control group and 9 out of 12 patients in the ETV group had cirrhosis. Interactions between preexisting cirrhosis and ETV treatment were not observed (P = 0.177).
Cumulative HCC incidence rates by risk scores are compared between the two cohorts in Fig. 4A-G. Figure 4A,B shows the risk scores developed by Yang et al.10 Figure 4C,D shows the risk scores developed by Yuen et al.11 Figure 4E-G shows the risk scores developed by Wong et al.12 All three risk score scales showed that ETV significantly reduced HCC incidence in patients with a higher risk (risk score ≥12, P = 0.006; risk score ≥82, P = 0.002; medium risk, P = 0.062; high risk, P < 0.001). Interactions between risk scores and ETV treatment were not observed (Yang et al.: P = 0.713, Yuen et al.: P = 0.267, Wong et al.: P = 0.265).
Discussion
Our study suggests that long-term ETV therapy would significantly suppress the development of HCC in HBV-infected patients when compared with HBV-infected patients in the control group. The treatment effect was more prominent among patients at high risk of HCC than those at low risk.
HBV has been previously shown to influence HCC development. Ikeda et al.20 reported that the cumulative HCC incidence rates among Japanese HBV patients were 2.1% at 5 years, 4.9% at 10 years, and 18.8% at 15 years among NA-naïve patients. Other studies, both from Japan and other countries, have reported a 5-year cumulative HCC incidence rate of 3.3% among chronic HBV, and 21.2% to 59% among cirrhosis patients.21, 22 The incidence of HCC varies significantly by country and ethnic group,4 which seems to be attributable to diverse exposure to HCC risk factors.
Carcinogenicity related to HBV infection is somewhat complex and multifactorial when compared with carcinogenicity related to HCV infection. Known HCC risk factors among HBV-infected patients include older age, male gender, cirrhotic status, diabetes mellitus, family history, alcohol consumption, AST, HBsAg, HBeAg, and genotype C.20, 23, 25 Chen et al.5 found a dose-response relationship between pretreatment serum HBV DNA levels and the development of HCC. Baseline ALT is another risk factor for HCC, as elevated ALT levels indicate an active immune response against HBV, resulting in repetitive hepatocyte injury.5 Our study corroborates these findings on these factors influence on HCC development.
The potential ability of ETV to reduce the risk of HCC is an additional example of a long-term NA treatment effect. Some studies have shown that ETV has low incidence of HCC but these studies did not have a control arm.9 A meta-analysis and a systematic review showed that NAs can reduce liver complications, including HCC.26, 27 Other studies have begun to show that control of sustained viral loads through drugs such as NAs is important in preventing long-term complications. Chen et al.28 showed that greater decreases in serum HBV DNA levels (<104 copies/mL) during follow-up were associated with a lower risk of HCC.
Our comparison among the PS-matched ETV-treated group, nonrescued LAM-treated patients, and the control showed that ETV is superior to LAM in HCC suppression. Kurokawa et al.29 showed that treatment with lamivudine for an average of 5 years reduced the incidence of HCC in HBV-infected cirrhosis patients, who showed sustained viral response at a median HBV DNA of <4.0 log copies/mL. Unfortunately, only 48% of the patients in this study achieved sustained viral response, while 51% developed lamivudine-resistant tyrosine-methionine-aspartate-aspartate mutation (YMDD mutation) during follow-up.29 Patients with drug resistance were reported to have a 2.6 times greater chance of developing long-term complications.26 A systematic review of 21 studies showed that HCC occurred more (2.3% versus 7.5%, P < 0.001) in nonresponding patients or in patients with viral breakthrough compared with those who experienced remission.28 On-treatment drug resistance could subject patients to a variable viral status. Suppression of HCC by NAs requires NAs that do not lead to drug resistance. Compared with other NAs, ETV shows minimal drug resistance. Our results showed that ~90% of the ETV-treated patients had sustained viral suppression at year 1, and that drug resistance was minimal (0.8%) during the median follow-up period of 3.2 years.
We found that the effect of ETV treatment in reducing the risk of HCC was more prominent among high-risk patients. This phenomenon was observed by examining the combination of parameters associated with the recently developed risk scores (Fig. 4). The published risk scores were developed mainly to create easy-to-use nomograms based on clinical characteristics to predict the risk of HCC in patients with HBV. These scales have been validated, and can accurately estimate the risk of HCC up to 10 years. The cutoff scores used in these studies were based on their sensitivity to detect HCC derived and validated with nontreated HBV cohorts. The importance of our study using these risk scales in our cohorts was to see the change in risk with the initiation of therapy. We found that the ETV treatment effect to reduce the risk of HCC was more prominent among cirrhosis and high-risk patients despite the lack of interactions between ETV treatment and preexisting cirrhosis or risk factors. The lower treatment effect among lower-risk patients was somewhat not surprising. HCC development among low-risk patients is generally rare, and therefore, the treatment effect may not have occurred in large enough numbers during the treatment period allotted in our study to be able to detect a difference. In addition, HCC development differs greatly by cirrhotic status and risk factors in the control group. The treatment effect of ETV to reduce HCC is probably more likely reflected among cirrhosis or high-risk patients. A study with a longer observation period and higher patient numbers might be necessary to examine this ETV treatment effect among low-risk patients. The development of a scoring system to predict treatment effect of HBV patients with different risk levels will be useful in determining the most appropriate timing of treatment initiation in clinical settings.
Study Limitations.
There were several limitations to our study. First, because our patients were recruited from one hospital, they might not have been representative of the general Japanese HBV population. Second, our control group included historically observed patients who entered the cohort long before the ETV group, resulting in treatment differences during the time gap. However, we used PS matching and a similar follow-up period between the two cohorts to minimize this bias. Third, our study was an observational study with patients having large demographic differences. Although we used a PS to match ETV-treated and control groups, our sample size did not take into account other unobserved confounding factors such as HCC family history, stage of cirrhosis, and comorbidities when determining associating factors for carcinogenesis in HBV. Finally, the observation period of the ETV group was relatively short, and patients in the ETV-treated cohort at 5 years consisted of only less than ~25% of the initial recruited patients. Because of this limitation, we censored patients who were followed for more than 5 years. The observed treatment effect would require confirmation over a longer period and a more complete follow-up.
Conducting a long-term study to examine the effect of antiviral therapy with HCC as the endpoint would be time-consuming and challenging. Such a study would require a large sample size and would, therefore, be costly. In addition, the increases in choices of therapy over time would make it difficult to conduct a long-term study using a single therapy. Owing to ethical issues, it would be difficult to recruit or follow a naïve, untreated cohort over an extended period of time. Because of these challenges, most studies have examined the relationship between antiviral treatment and the risks of HCC involved older drugs, lacked a control group, or were of relatively short duration.
Consequently, the association between antiviral treatment and carcinogenesis is inferential and requires additional confirmatory studies.
In conclusion, in our study we observed the effect of HCC risk among HBV-infected patients treated by ETV by comparing them with a group of NA-naïve patients. We followed these Japanese patients for a relatively long period of time and compared them with a large pool of untreated control patients. In this long-term study among Japanese patients, ETV significantly reduced the incidence of HCC among chronic HBV-infected patients, and was more prominent among patients at higher risk for HCC.
|
|
| |
| |
|
|
|