| |
Idenix Pharmaceuticals Provides Update on IDX184 and IDX19368 Development Programs, stops 2 drugs' development, starts another
|
| |
| |
press release
Feb. 4, 2013, 4:05 p.m. EST
CAMBRIDGE, Mass., Feb 04, 2013 (GLOBE NEWSWIRE via COMTEX) -- Idenix Pharmaceuticals, Inc., a biopharmaceutical company engaged in the discovery and development of drugs for the treatment of human viral diseases, today announced the Company has elected not to continue its clinical development program for IDX184, a nucleotide polymerase inhibitor in phase IIb testing for the treatment of hepatitis C virus (HCV) infection, or to continue its development of IDX19368, an HCV nucleotide polymerase inhibitor for which the Company had previously filed an IND but had not initiated patient dosing. In August 2012, the U.S. Food and Drug Administration (FDA) placed IDX184 on partial clinical hold and IDX19368 on clinical hold due to cardiac adverse events seen in a competitor's phase II clinical trial of BMS-986094. All three drug candidates are 2'-methyl guanosine nucleotide prodrugs. In December, Idenix completed the submission of requested cardiac safety data for IDX184 to the FDA. Idenix has found no evidence of severe cardiac findings to date. In February, the FDA communicated that the IDX184 and IDX19368 programs would remain on clinical hold, and, as a result, the Company has determined it will not continue the development of these programs.
"Although we are choosing not to continue our IDX184 and IDX19368 programs, we intend to maintain our strong presence in developing nucleotide polymerase inhibitors for HCV based on our broad discovery platform," said Ron Renaud, Idenix's President and Chief Executive Officer. "We are completing IND-enabling studies for a uridine nucleotide analog, which is in a sub-class of nucleotide polymerase inhibitors distinct from IDX184, IDX19368 and BMS-986094. We anticipate filing an IND for this next-generation compound during the first half of 2013, and we also plan to continue to advance other preclinical nucleotide prodrugs in earlier-stage development."
Mr. Renaud continued, "Further, we are pleased with the progress of IDX719, our potent, pan-genotypic NS5A inhibitor for HCV. In January 2013, we entered into a non-exclusive collaboration with Janssen Pharmaceuticals, Inc. for the development of all-oral direct-acting antiviral (DAA) HCV combination therapies incorporating IDX719. Following an initial drug-drug interaction study to begin in the first quarter of 2013 and pending approval from regulatory authorities, we expect to begin the first phase II study under this program of a two-DAA regimen, including IDX719."
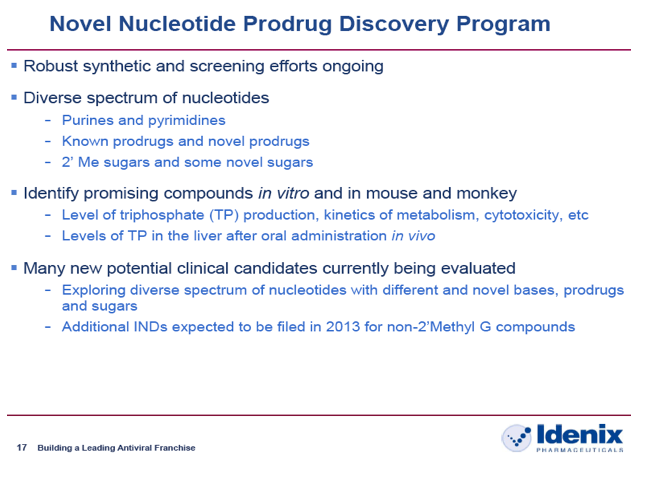
Idenix: "Novel Nucleotide Prodrug Discovery Program: Additional INDs expected to be filed in 2013 for non-2'Methyl G compounds"
|
|
| |
| |
|
|
|