| |
New FDA Approval Process: "Breakthrough therapy"
|
| |
| |
Download the PDF here
First 'breakthrough' drugs designated, but dilution worries linger
(from Jules: what about HCV, fast-tracking & accelerated approval for new HCV drugs, the FDA needs to do a better fast-tracking of new HCV therapies; new HCV orals are breakthrough drugs too!)
Nature Medicine Feb 2013
The US Food and Drug Administration already has numerous ways it can speed up the market authorization of new medicines, ranging from 'accelerated approvals' to 'priority reviews' to its fast-track program. Even so, sometimes the existing mechanisms for speeding drugs to market-which typically require data from the traditional three phases of drug development-aren't fast enough for the millions of patients in desperate need of new medicines. With the first so-called 'breakthrough therapy' designation awarded last month, patient advocates see a signal that the FDA will green-light exceptional drugs more quickly with this new regulatory pathway, but some worry that if the designation is overused its value could be diminished.
On 6 January, Vertex Pharmaceuticals of Cambridge, Massachusetts, announced that two of its cystic fibrosis therapies-Kalydeco (ivacaftor) taken alone or in combination with an experimental agent called VX-809-had received the first breakthrough designations under the FDA's new program, which was codified into law in July 2012 as part of the reauthorization of the Prescription Drug User Fee Act.
Kalydeco, the first available drug that targets the defective protein responsible for cystic fibrosis, was approved last year after a lightning-quick three-month review for the 4% of people with cystic fibrosis who harbor a particular mutation in the cystic fibrosis transmembrane conductance regulator (CFTR) gene known as G551D and are older than 6. Vertex is now seeking to gain broader approvals for the use of Kalydeco in younger children with the G551D mutation as well as those with other mutations that affect the CFTR protein in similar ways. Vertex is also advancing Kalydeco together with VX-809 for people with the most common type of cystic fibrosis mutation, known as F508del. "We see this new designation as a great opportunity to work with [the FDA] closely toward a mutual goal of bringing these potentially important medicines to the people who need them as soon as possible," says Megan Goulart, Vertex's senior manager of cystic fibrosis product communications and patient advocacy.
Despite granting the first breakthrough designations for Vertex's products, regulators are still fleshing out what exactly constitutes a breakthrough therapy-guidance documents are expected to be published by January 2014. However, last summer's law described it as something that can "treat a serious or life-threatening disease or condition and for which preliminary clinical evidence indicates that the drug may demonstrate substantial improvement over existing therapies."
Percy Ivy, associate chief of the Investigational Drug Branch at the US National Cancer Institute's Cancer Therapy Evaluation Program in Rockville, Maryland, likens the definition of a breakthrough drug to US Supreme Court Justice Potter Stewart's notorious 1964 definition of pornography. "You're just going to know it when you see it," Ivy says.
Of course, such nonstandard development programs existed before the breakthrough label. A study conducted by the Connecticut-based National Organization for Rare Disorders (NORD) looked at all therapies for diseases other than cancer that were approved as orphan drugs between 1983 and mid-2010. It concluded that two-thirds of approvals involved some degree of flexibility from the conventional data requirements, the majority on a case-by-case basis that fell outside the formal systems such as accelerated approvals that allow for scientific discretion in assessing effectiveness evidence. Nonetheless, the breakthrough designation adds some "predictability and transparency" to how and when flexibility will be applied, notes Mary Dunkle, vice president for communications at NORD. "We would rather have the system work with pathways that are documented and everyone is aware of," she says.
Break on through (to the other side)
What sets the breakthrough designation apart from other expedited drug development mechanisms-all of which have been in place at the FDA for at least 20 years-is the requirement of early clinical data demonstrating an unprecedented effect (see 'Drug development in the fast lane'). Fast-track designation, for example, can be granted off the back of promising preclinical data; accelerated approval status has more to do with surrogate trial endpoints. And although companies with fast-tracked drugs will receive earlier and more frequent communication from the FDA, they won't get the 'all hands on deck' approach that is promised by the breakthrough designation. For breakthrough therapies, senior FDA managers and reviewers are expected to work closely with drug sponsors to design collaborative, multidisciplinary development plans that hasten timelines to approval and minimize the number of patients exposed to less efficacious treatments or placebos.
Table 1: Drug development in the fast lane: FDA approaches to expedited approval.
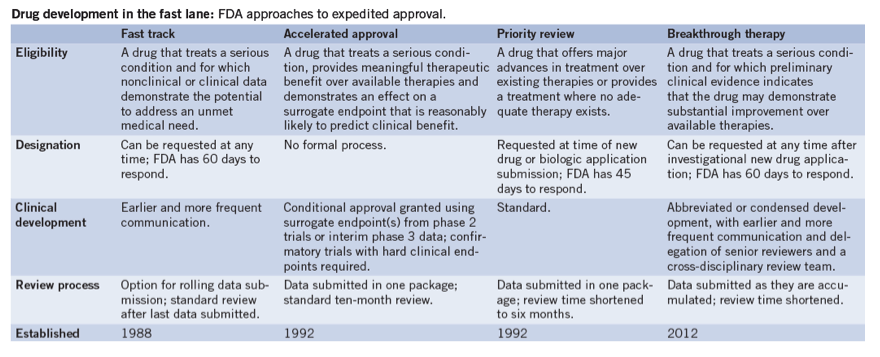
"This is really meant to signal from the agency that if you have a drug that shows a really unprecedented activity early on, they want to work with you to find the best course forward, rather than have you go it alone," says Jeff Allen, executive director of Friends of Cancer Research, a think tank and advocacy organization based in Washington, DC, that has actively championed the new breakthrough pathway.
Ultimately, Ivy expects patients to benefit as much as, if not more than, drug sponsors. Currently, individuals who are desperately seeking unapproved treatments but cannot enroll in a clinical trial must petition the FDA for a 'special exception use', yet drug companies are not always so keen to provide investigational medicines outside the confines of a controlled study. The breakthrough designation should increase drug access earlier on. "In the end," Ivy says, "I think the driver, goal and motivation for breakthrough therapies is to make treatments more widely accessible to patients who don't have other options."
However, onlookers say they hope the influence of a breakthrough designation won't be weakened by the FDA assigning it too frequently. "Like fast-track designations, I suspect they're going to give too many," says Greg Dombal, chief operating officer of Halloran Consulting Group, a Boston area firm that specializes in the life sciences industry. "And, in practice, its value could be slightly watered down because there are going to be a lot of things in there that shouldn't have that designation."
|
|
| |
| |
|
|
|