| |
Moderate, excessive or heavy alcohol consumption: each is significantly associated with increased mortality in patients with chronic hepatitis C
|
| |
| |
Download the PDF here
.....NHANES survey, 218 with HCV, 32 deaths due to liver diseases....."moderate drinkers" 1-2 drinks per day (1-19 g/alcohol/day) HCV was associated with 2-fold increased risk for overall mortality (2.33-fold) compared HCV-negs, 2-3 drinks increased 7-fold, and 3 or more daily drinks 3.5 fold increased risk...the purpose of study was to assess the impact of alcohol......people with HCV but no history of alcohol use all-cause mortality increased 2.52-fold
Alimentary Pharmacology & Therapeutics April 2013
Z. M. Younossi et al
Center for Liver Diseases,
Department of Medicine, Inova Fairfax
Hospital,
The impact of different amounts of alcohol consumption on CH-C mortality
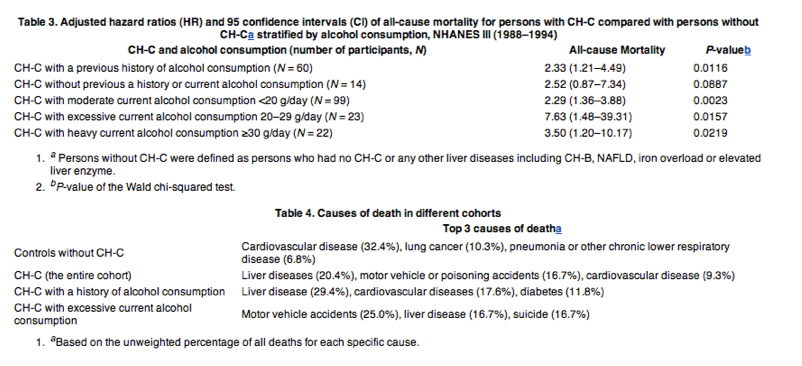
The impact of CH-C on the mortality outcomes stratified by levels of alcohol consumption is summarized in Table 3.
"These results should inform clinicians to advocate complete alcohol abstinence in patients with CH-C. Additionally, our study should inform health care policy makers to address CH-C infection at the population level and recognize that CH-C will not only increase the risk for liver-related mortality but also the risk for overall mortality. This recognition of CH-C as an important cause of all-cause mortality at the population level should prompt the availability of resources to address this important public health issue both nationally and globally."
"This study uses the population data collected as a part of NHANES III surveys and the associated Mortality Linked Files to assess the impact of CH-C and the combination of CH-C with alcohol consumption on overall mortality and cause-specific mortality in US general population. Our data show that patients with CH-C are at increased risk for overall mortality and liver-related mortality. Although the increased risk of overall mortality in CH-C has been controversial, this study supports this increased risk"
"The main outcomes in this study were all-cause mortality, liver-related mortality and cardiovascular mortality. The NHANES III Linked Mortality File provides mortality follow-up data from the date of NHANES III survey participation (1988-1994) through 31 December 2006.[10, 14] Mortality ascertainment is based on the results from a probabilistic match between NHANES III and the National Death Index (NDI) death certificate records. In addition to mortality status, the linked file contains months of follow-up from examination date as well as the Underlying Cause of Death 113 (UCOD_113) code to recode all deaths according to ICD-9 and ICD-10 criteria.[14] Liver-related mortality (UCOD_113 15, 24, 93-95) included causes of death such as viral hepatitis, hepatocellular carcinoma, alcoholic liver disease, and other chronic liver disease and cirrhosis. Cardiovascular mortality (UCOD_113 58-63, 67, 70-74) covered causes of death such as ischaemic heart diseases, heart failure, atherosclerosis, cerebrovascular diseases, aortic aneurysm and other diseases of arteries, arterioles and capillaries."
"Additionally, our data confirm that CH-C is independently associated with increased risk for liver-related mortality at the population level. Given the estimated 4-5 million individuals infected with HCV in the United States and 130-140 million people infected with HCV worldwide,[1, 3, 18] these findings can have important national and global implications"
"415 (3.39%) deaths due to cardiovascular diseases and 32 (0.27%) deaths due to liver diseases."
"Compared with participants without CH-C, participants with CH-C were more likely to have excessive alcohol consumption, smoke cigarettes, to be male, younger than 45 years, non-Hispanic black, and have diabetes or insulin resistance"
"For participant without excessive alcohol consumption (1-3 drinks/day), having CH-C was associated with increased risk of all-cause mortality (HR: 2.44, 95% CI: 1.59-3.75, P < 0.01) and liver-related mortality (HR: 74.25, 95% CI: 19.62-280.92, P < 0.01)."
"Among participants with a history of alcohol consumption (but currently none), those with CH-C had over two-fold increased risk of overall mortality as compared with those without the disease (HR: 2.33, P = 0.01). For participants who actively consumed moderate amounts of alcohol (1-19 g/day), CH-C was also associated with two-fold increased risk of overall mortality [HR = 2.29 (1.36-3.88), P = 0.01]. However, CH-C was associated with over seven-fold increased risk of overall mortality for those individuals who were consuming 20-29 g of alcohol per day (HR = 7.63, P = 0.02), and a three-and-a-half-fold increased risk of overall mortality for those consuming ≥30 g of alcohol per day (HR = 3.50, P = 0.02) (Table 3)."
"It is important to note that for individuals with CH-C, liver disease was the top causes of death, accounting for 20.4% of all deaths. On the other hand, among participants without CH-C or any other liver diseases, the top cause of death was cardiovascular disease, which accounted for 32.5% of all deaths (Table 4)."
Summary
Background
The impact of moderate alcohol consumption on long-term outcomes of chronic hepatitis C (CH-C) infected patients remains controversial.
Aim
To assess the impact of moderate alcohol consumption on long-term outcomes of CH-C patients using population-based data.
Methods
Data were obtained from the Third National Health and Nutrition Examination Survey (NHANES III)-mortality linked files. Alcohol consumption was estimated as grams/day. Multivariate Cox proportional hazards model was utilized to assess the effects of CH-C and alcohol consumption on mortality (all causes, cardiovascular disease, and liver disease).
Results
A total of 8985 participants were included as the study cohort. Of these, 218 had CH-C. The follow-up time was 162.95 months for CH-C and 178.27 months for controls. CH-C patients had increased risk for both overall mortality and liver-related mortality. CH-C patients with excessive alcohol consumption had even higher risks for overall mortality and liver-related mortality. The risk of overall mortality associated with CH-C increased with moderate alcohol consumption of 1-19 g/day and heavy alcohol consumption ≥30 g/day.
Conclusion
Although chronic hepatitis C is associated with increased risks for overall and liver-related mortality, these risks are even higher for patients consuming moderate and excessive amounts of alcohol.
Introduction
Chronic hepatitis C (CH-C) is a major cause of chronic liver disease, cirrhosis and hepatocelluar carcinoma (HCC). CH-C is associated with tremendous burden on the health and the well-being of the population.[1, 2] Although the incidence of new cases of hepatitis C virus (HCV) infection has fallen,[3] over the next few decades, a large cohort of actively infected CH-C patients is expected to be responsible for a significant rise in the number of cases of HCC and decompensated cirrhosis.[4, 5] In this context, CH-C will continue to increase its clinical and economic burden and will overtake other important chronic infectious diseases, including HIV in terms of mortality.[6]
Despite this projected threat from HCV, not all patients progress to cirrhosis and develop HCV-related complications.[1, 3] There are a number of factors that affect progression of liver disease in patients with CH-C.[7] Of these, excessive alcohol consumption has been recognized as an important factor.[8, 9] The impact of moderate alcohol consumption in patients chronically infected with HCV has not been fully understood. The aim of this study was to assess the impact of excessive and moderate alcohol consumption on the overall, liver-related and cardiovascular mortality of patients with CH-C.
Methods
To assess the impact of alcohol consumption on CH-C mortality, we used the Third National Health and Nutrition Examination Survey (NHANES III) and the NHANES III linked mortality file.[10] The survey was conducted from 1988 through 1994 using a complex, multi-stage, stratified, clustered sample design to obtain a representative sample of the total civilian, non-institutionalized US population. It includes an in-home interview for demographic and basic health information, a health examination in a mobile examination center, and laboratory/imaging tests (including ultrasonography of the gallbladder and liver). Public use data files were obtained from the NHANES Website (http://www.cdc.gov/nchs/nhanes.htm). The study was approved by the Inova Institutional Review Board.
Study population
During NHANES III, 33 994 participants were interviewed in their homes, in which standardized questionnaires were used to obtain self-reported data on age, gender, race or ethnicity, smoking, and prevalent medical conditions.[10] The present study was restricted to adult participants (aged 20-74 years at the time of the examination). Age was categorized into four groups: 20-44, 45-54, 55-64, and 65-74. Four major race/ethnic groups were reported including non-Hispanic white, non-Hispanic black, Mexican American and 'Other,' which included all Hispanics who were not Mexican American and also all non-Hispanics from racial groups other than white and black. A positive smoking history was defined as ongoing smoking or smoked at least 100 cigarettes in life. For participants who underwent health examinations, blood and urine specimens were obtained, and a number of body measurements and tests were performed including height, weight, waist circumference, systolic and diastolic blood pressure, glucose tolerance tests, diabetes tests, general biochemistry tests, HCV antibody tests, and ultrasonography. Analysis was restricted to persons with complete data on demographics (age, gender, race/ethnicity), history of smoking and alcohol consumption, history of type II diabetes, body mass index, waist circumference, blood pressure measured at the time of examination, as well as gradable hepatic ultrasound video images for hepatic steatosis assessment. The following tests also were required for all individuals in this study: serum glucose, triglyceride, high-density lipoprotein, aspartate aminotransferase, alanine transaminase, transferrin saturation levels, and viral hepatitis serologies for hepatitis B virus (HBV) and hepatitis C virus (HCV).
Chronic hepatitis C (CH-C)
All serum from participants was tested for antibody to hepatitis C virus (anti-HCV) and positive anti-HCV tests were confirmed using HCV RNA by polymerase chain reaction (PCR). Participants with positive HCV RNA were considered to have chronic hepatitis C (CH-C). In the current study, only patients with established CH-C in absence of CH-B or iron overload were included. The unexposed group (Controls) consisted of individuals without CH-C, CH-B, NAFLD, iron overload, or elevated liver enzymes.
Exposure to alcohol consumption
Alcohol consumption was assessed during the alcohol and drug assessment component of the NHANES III medical examination.[10] Participants were asked if they had at least 12 drinks of alcohol in the past 12 months as well as life time. Persons who reported no alcohol drinking in their entire lives were considered to be 'never drinkers'. Historical drinkers were defined as persons who reported drinking at least 12 drinks of alcohol in entire life, but none in the past 12 months. Current drinkers were defined as persons who reported at least 12 drinks of alcohol in the past 12 months.
The amount of current alcohol consumption was determined from self-reported number of days with alcohol drinking and the average number of drinks per day when they drank alcohol in the past 12 months. As defined by NHANES, one drink which was equivalent to 10 g ethanol represented 12-oz of beer, 4-oz of wine or 1-oz of liquor. Average daily alcohol consumption was estimated by: the number of drinks on a drinking day x 10 g x the number of drinking days over the past 12 months/365.
Moderate alcohol consumption was defined as 1-19 g of alcohol consumption per day and excessive alcohol consumption was defined as average daily alcohol consumption of 20 g or greater.[11] A sub-category of excessive alcohol consumption was considered as heavy alcohol consumption, which was defined as 30 g or more of alcohol consumption per day.
Participants who did not answer the alcohol consumption questions and those who reported drinking alcohol in the past 12 months but did not report the frequency or amount of alcohol consumption were excluded from this study.
Study definitions
1. Diabetes mellitus type 2 (DM) was defined as a fasting glucose value of 126 mg/dL or greater or the use of oral hypoglycemics and/or insulin.
2. Insulin resistance (IR) was evaluated using the homeostasis of model assessment score (HOMA), which was calculated using the formula: fasting serum insulin (μU/mL) x fasting plasma glucose (mmol/L)/22.5.[12] HOMA-IR was defined as a HOMA score of 3.0 or greater. DM and IR were combined as one variable DM/IR in our data analysis.
3. Hypertension was defined as a systolic blood pressure of 140 mmHg or greater, diastolic blood pressure of 90 mmHg or greater, or being on oral antihypertensive medications.
4. Obesity was defined as a body mass index greater than 30 or a waist circumference more than 102 cm in men and more than 88 cm in women.
5. All serum was tested for core antibody to hepatitis B virus (anti-HBC). Serum testing positive for anti-HBC were tested further for the hepatitis B surface antigen (HBsAg). Chronic Hepatitis B (CH-B) was presumed in individuals with positive HBsAg.
6. Elevated serum transferrin saturation is a commonly used indicator for a predisposition of iron overload. Potential iron overload is defined as serum transferrin saturation greater than 50%.
7. Elevated liver enzyme was defined as serum alanine aminotransferase level greater than 40 U/L or aspartate aminotransferase level greater than 37 U/L in men and alanine aminotransferase or aspartate aminotransferase level greater than 31 U/L in women.
8. Between 2009 and 2010, the hepatic steatosis (fatty liver) was assessed by reviewing ultrasound video images originally obtained in NHANES III between 1988 and 1994.[13] Nonalcoholic fatty liver disease (NAFLD) was defined as presence of moderate-to-severe hepatic steatosis from the ultrasound examination in the absence of any other evidence of chronic liver disease such as ALD, CH-B, CH-C and iron overload.
Follow-up for mortality
The main outcomes in this study were all-cause mortality, liver-related mortality and cardiovascular mortality. The NHANES III Linked Mortality File provides mortality follow-up data from the date of NHANES III survey participation (1988-1994) through 31 December 2006.[10, 14] Mortality ascertainment is based on the results from a probabilistic match between NHANES III and the National Death Index (NDI) death certificate records. In addition to mortality status, the linked file contains months of follow-up from examination date as well as the Underlying Cause of Death 113 (UCOD_113) code to recode all deaths according to ICD-9 and ICD-10 criteria.[14] Liver-related mortality (UCOD_113 15, 24, 93-95) included causes of death such as viral hepatitis, hepatocellular carcinoma, alcoholic liver disease, and other chronic liver disease and cirrhosis. Cardiovascular mortality (UCOD_113 58-63, 67, 70-74) covered causes of death such as ischaemic heart diseases, heart failure, atherosclerosis, cerebrovascular diseases, aortic aneurysm and other diseases of arteries, arterioles and capillaries. As HIV screening results were not released in NHANES III data for public use, to separate the effects of co-infection of HCV and HIV, individuals who died of human immunodeficiency virus (HIV) (UCOD_113 16) were presumed to have HIV infection and were excluded. Individuals without available mortality follow-up data were also excluded from the study.
Statistical analysis
We compared the baseline characteristics of participants by the status of CH-C using χ2-test for independence. Periods of risk of death were defined in months for each participant between NHANES III examination date and the date of death or the end of follow-up (31 December 2006). If an individual did not die or died from causes other than the event of interest, his survival time was censored.
Cox proportional hazards model was used to estimate hazard ratios and 95% confidence intervals for deaths from all causes, cardiovascular disease and liver disease by status of CH-C. To examine the amount of alcohol consumption on the survival of persons with CH-C, stratified analyses by levels of alcohol consumption were conducted Each model was adjusted for major demographic and clinical confounders including age, gender, race/ethnicity, smoking history, obesity, diabetes/insulin resistance and hypertension. Statistical significance was set at P < 0.05.
NHANES III is based on a complex multistage probability sample design. The sampling weights incorporate the differential probabilities of selection and include adjustments for noncoverage and nonresponse. Sample weights together with stratification and clustering were incorporated into our analysis to estimate variances and test for statistical significance.[10] All analyses were performed using standalone SUDAAN 10.0 (RTI International, Research Triangle Park, NC, USA).
Results
After applying the exclusion criteria, a total of 8985 participants remained in the analytical sample. Table 1 summarizes the characteristics of participants according to their diagnosis.
Compared with participants without CH-C, participants with CH-C were more likely to have excessive alcohol consumption, smoke cigarettes, to be male, younger than 45 years, non-Hispanic black, and have diabetes or insulin resistance. Previous studies have shown that participants' age, gender, race/ethnicity, smoking status and disease conditions including obesity, DM/IR and hypertension were associated with mortality (7). Therefore, multivariate Cox proportional hazards model was utilized to evaluate the independent effect of each diagnosis on all-cause mortality and cause-specific mortality while adjusting for possible confounding effects of these variables. Exploratory analyses revealed that the effect of obesity on mortality varied across age groups; therefore, an interaction between age and obesity was included in the multivariate model.
The impact of chronic hepatitis C on mortality
The median follow-up was 162.95 months for patients with CH-C, and 175.49 months for controls. A total of 1320 (11.37%) participants died at the end of the follow-up, including 415 (3.39%) deaths due to cardiovascular diseases and 32 (0.27%) deaths due to liver diseases.
For CH-C patients, the cumulative mortality rate was 19.09% (54 deaths) for all-cause death, 1.66% (5 deaths) for cardiovascular death and 6.37% (11 deaths) for liver-related death. In comparison, the cumulative mortality rate for controls was 11.22% (1266 deaths) for all-cause death, 3.43% (410 deaths) for cardiovascular death, and 0.15% (21 deaths) for liver-related death.
The unadjusted analyses showed that compared with controls, patients with CH-C had significantly higher risk for all-cause mortality (HR: 1.91, 95% CI: 1.16-3.15, P = 0.01) and liver-related mortality (HR: 49.52, 95% CI: 12.37-198.26, P < 0.01), but not cardiovascular mortality (HR: 0.53, 95% CI: 0.17-1.67, P = 0.28).
The impact of excessive alcohol consumption and chronic hepatitis C on mortality
The combined effects of CH-C and excessive alcohol consumption on mortality outcomes were evaluated by including an interaction term of these two factors in the multivariate Cox proportional hazard model, the results are summarized in Table 2.
For participant without excessive alcohol consumption, having CH-C was associated with increased risk of all-cause mortality (HR: 2.44, 95% CI: 1.59-3.75, P < 0.01) and liver-related mortality (HR: 74.25, 95% CI: 19.62-280.92, P < 0.01). Similar to the unadjusted results; CH-C without excessive alcohol use was not associated with cardiovascular mortality (HR: 0.71, 95% CI: 0.23-2.21, P = 0.55).
On the other hand, for participants with excessive alcohol use, having CH-C dramatically increased the risk of all-cause mortality (HR: 5.12, 95% CI: 1.97-13.28, P < 0.01), liver-related mortality (HR: 183.74, 95% CI: 15.98-infinity, P < 0.01), as well as a trend in increasing the risk of cardiovascular mortality (HR: 3.34, 95% CI: 0.55-20.50, P = 0.19) (Table 2).
The impact of different amounts of alcohol consumption on CH-C mortality
The impact of CH-C on the mortality outcomes stratified by levels of alcohol consumption is summarized in Table 3.
Among participants with a history of alcohol consumption (but currently none), those with CH-C had over two-fold increased risk of overall mortality as compared with those without the disease (HR: 2.33, P = 0.01). For participants who actively consumed moderate amounts of alcohol (1-19 g/day), CH-C was also associated with two-fold increased risk of overall mortality [HR = 2.29 (1.36-3.88), P = 0.01]. However, CH-C was associated with over seven-fold increased risk of overall mortality for those individuals who were consuming 20-29 g of alcohol per day (HR = 7.63, P = 0.02), and a three-and-a-half-fold increased risk of overall mortality for those consuming ≥30 g of alcohol per day (HR = 3.50, P = 0.02) (Table 3).
It is important to note that for individuals with CH-C, liver disease was the top causes of death, accounting for 20.4% of all deaths. On the other hand, among participants without CH-C or any other liver diseases, the top cause of death was cardiovascular disease, which accounted for 32.5% of all deaths (Table 4).
Discussion
This study uses the population data collected as a part of NHANES III surveys and the associated Mortality Linked Files to assess the impact of CH-C and the combination of CH-C with alcohol consumption on overall mortality and cause-specific mortality in US general population. Our data show that patients with CH-C are at increased risk for overall mortality and liver-related mortality. Although the increased risk of overall mortality in CH-C has been controversial, this study supports this increased risk.[15-17]
Additionally, our data confirm that CH-C is independently associated with increased risk for liver-related mortality at the population level. Given the estimated 4-5 million individuals infected with HCV in the United States and 130-140 million people infected with HCV worldwide,[1, 3, 18] these findings can have important national and global implications.[5]
In this study, we also assessed the interaction between CH-C and alcohol consumption. Previous studies have reported that excessive alcohol consumption can be associated with increased risk of liver-related mortality, but not with the increased risks of overall mortality or cardiovascular mortality.[19] Our study also shows that having CH-C in individuals who excessively consume alcohol can dramatically increase the risk of all-cause mortality, liver-related mortality and potentially, cardiovascular mortality. In fact, in patients with CH-C, this increased risk for overall mortality, and liver-related mortality was further amplified with increasing amounts of alcohol consumption. Although alcohol consumption in excess of 40-50 g/day has previously been associated with an increased risk of liver-related mortality,[20] this is the first study documenting the combined effects of alcohol consumption and CH-C on both overall mortality and liver-related mortality. This is especially important for CH-C patients who consume moderate amounts of alcohol (<20 g per day). Although the detrimental effect of 'moderate alcohol use' in CH-C patients has been clinically suspected,[21] this population-based study provides further evidence to support this association. In our study, moderate alcohol consumption in patients with CH-C does not seem to have a potential 'cardio protective' effect. On the contrary, the negative impact of alcohol consumption related to HCV-related liver disease may outweigh any potential cardiac benefits.
Despite its long-term follow-up and in-depth clinical and mortality data, our study does have some weaknesses. The most important weakness is the relative small sample size of patients with CH-C, which may have led to our inability to show some important potential associations as well as being responsible for the large confidence interval seen in some of the hazard ratios. Nevertheless, the in-depth nature of this population-based study and the long-term follow-up mortality data make this study quite unique and important. Future research can include the incorporation of similar epidemiological and natural history data of this same population in one comprehensive report. Additionally, a meta-analysis that will combine some of the similar studies assessing interaction between HCV, alcohol consumption and mortality will be important.
In summary, our study finds that patients with CH-C are at increased risks for both liver-related mortality and overall mortality. This risk increases in CH-C patients who consume alcohol excessively and potentially moderately. These results should inform clinicians to advocate complete alcohol abstinence in patients with CH-C. Additionally, our study should inform health care policy makers to address CH-C infection at the population level and recognize that CH-C will not only increase the risk for liver-related mortality but also the risk for overall mortality. This recognition of CH-C as an important cause of all-cause mortality at the population level should prompt the availability of resources to address this important public health issue both nationally and globally.
|
|
| |
| |
|
|
|