| |
Long-term antiretroviral therapy initiated during primary HIV-1 infection is key to achieving both low HIV reservoirs and normal T cell counts
|
| |
| |
Download the PDF here
Download the PDF here
Journal of Antimicrobial Chemotherapy Advance Access published January 25, 2013
Laurent Hocqueloux1 *, Ve' ronique Avettand-Fenoe l2,3 , Sophie Jacquot4 , Thierry Prazuck1 , Eric Legac5 , Adeline Me'lard2,3 , Mohamadou Niang1 , Catherine Mille1 , Gwenae l Le Moal6 , Jean-Paul Viard3,7 and
Christine Rouzioux2,3 on behalf of the AC32 (Coordinated Action on HIV Reservoirs) of the Agence Nationale de Recherches sur le Sida et les He'patites Virales (ANRS)
1Service des Maladies Infectieuses et Tropicales, Centre Hospitalier Re'gional, Orle'ans, France; 2Laboratoire de Virologie, Ho pital Necker-Enfants Malades, APHP, Paris, France; 3EA 3620, Universite' Paris-Descartes Sorbonne Paris Cite', Faculte' de Me'decine,
Paris, France; 4Laboratoire de Mathe'matiques - Analyse, Probabilite's, Mode'lisation (MAPMO), Universite' d'Orle'ans, Orle'ans, France; 5Laboratoire de Biologie, Centre Hospitalier Re'gional, Orle'ans, France; 6Service des Maladies Infectieuses et Tropicales, Ho pital de la
Mile'trie, Poitiers, France; 7Service des Maladies Infectieuses, APHP, Ho tel Dieu, Paris, France
"......In conclusion, we show that primary HIV infection represents a critical period (possibly a unique opportunity) during which cART can induce a profound decrease in the viral reservoir together with rapid optimal immune restoration. In this setting, long-term treatment provides the most favourable chance of obtaining the lowest level of residual viral reservoir and better immune reconstitution. Regarding patients with a later diagnosis of HIV infection, we show that cART initiation before the CD4+ T cell count falls below 500 cells/mm3 may still induce OVIR status, whereas delaying treatment greatly reduces the chances of reaching this status, even after up to 14 years of sustained viral suppression. Our findings also support public health programmes designed to promote HIV testing and treatment ('test and treat'), especially among highly exposed populations, as early treatment may not only limit the risk of transmission,47 but may also benefit the patients themselves."
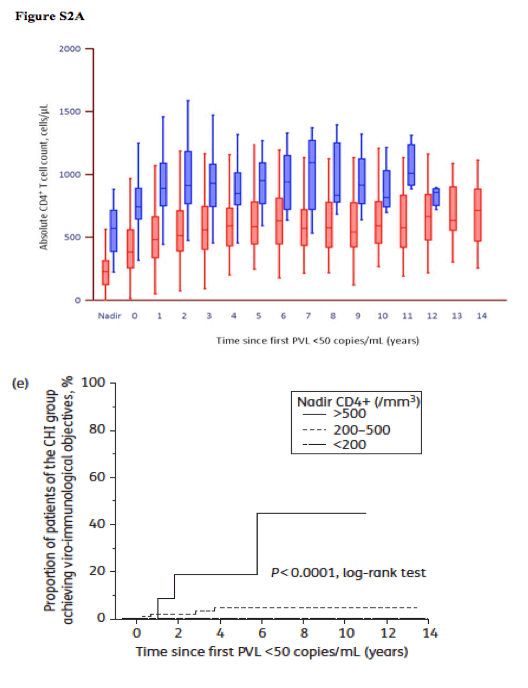
"Both PHI and CHI groups had similar increases in the three immunological markers. Nevertheless, final values at the LOCF differed markedly, always in favour of the PHI group: the median CD4+ T cell count was 883 cells/mm3 versus 619 cells/mm3 (P< 0.0001); the median CD4+ T cell percentage was 41% versus 31% (P< 0.0001); and the median CD4+/CD8+ ratio was 1.31 versus 0.77 (P< 0.0001). All three markers normalized in 28/35 PHI patients (80%) and 72/272 (26%) CHI patients (P< 0.0001). The CD4+ T cell count was above the threshold of 500 cells/mm3 in 34/35 (97%) PHI patients and 191/272 (70%) CHI patients (P = 0.0002). Regardless of the duration of undetectable PVL, the PHI group always showed better T cell reconstitution on cART than the CHI group (Figure S2, available as Supplementary data at JAC Online)."
"Altogether, these differences in HIV DNA decay between the PHI and CHI groups strongly suggest that early treatment prevents and/or decreases the establishment of large viral reservoirs in resting cells with long half-lives, while cART has a very low impact on infected resting cells that are well stabilized at the chronic phase. Additionally, cART initiated during PHI could preserve immune functions (innate and adaptive), enabling better immune reconstitution and hence more efficient clearance of the pool of infected cells, even during the second (slow) phase of HIV DNA decay......While we observed long-term advantages to being treated since PHI (versus CHI) for all immunological parameters taken separately, the comparison was even more striking when we looked at the combination of all three markers of immune reconstitution. Like Kelley et al.,38 we not only found that some CHI patients never achieved CD4+ T cell counts above the threshold of 500 cells/mm3, but we also showed that the majority of them never achieved normal T cell counts-particularly a normal CD4+/CD8+ ratio-even after 14 years of antiretroviral therapy. In contrast, 90% of the group treated since PHI was able to reach that goal after 6 years of therapy, and almost all within a decade of therapy......Several authors have recently postulated that early treatment could lead to a viro-immunological status mimicking that of Elite controllers"
"patients in the PHI group had heavily symptomatic primary infection, a feature associated with less-favourable immuno-virological parameters and disease outcomes.46 Finally, this reinforces the benefit of long-term cART initiated during primary HIV-1 infection, as it achieved the better observed immune reconstitution and depletion of HIV reservoirs, despite unfavourable immuno-virological parameters before treatment."
"This study is, to our knowledge, the first to report the very long-term (≥10 years) impact of continuous cART on viral reservoirs and immune reconstitution in a large prospective cohort, comparing patients treated since PHI and CHI. It emphasizes the large viro-immunological benefit of initiating treatment at the PHI phase; 54% of patients who started treatment during PHI achieved both a very low blood viral reservoir (HIV DNA level <2.3 log10 copies/106 PBMCs) and normal values of all three immunological parameters (CD4+ count and percentage, and CD4+/CD8+ ratio). In contrast, only 3% of patients achieved these results when cART was started later, i.e. during the chronic phase, and interestingly, these patients were characterized by a high CD4+ nadir (>500 cells/mm3). Nevertheless, such benefits should be balanced with the risk and cost of a longer exposure to antiretroviral drugs.24"
"Recently Ananworanich et al.33 demonstrated that HIV DNA levels increase gradually during the first weeks after acute infection, suggesting that the earlier cART is introduced, the lower the HIV DNA level is to be decreased. This observation was supported by Archin et al.,34 who showed that cART reduced the generation of latently infected CD4+ T cells in patients treated during acute seronegative HIV-1 infection. Nevertheless, in our study only 10% of patients treated at PHI were seronegative"
"In the PHI group there is a fast initial decay after cART initiation, with a half-life of 113 days, similar to those previously reported......we found a slow decay in the second phase for up to a decade of successful therapy.......The slow HIV DNA decay we found during the second phase of follow-up was significantly stronger in patients treated since PHI than in those treated since CHI"
"The potential benefits of combining sustained control of viral replication, a small viral reservoir and an optimal immune reconstitution are highlighted by studies of rare natural (Elite) or post-treatment controllers. Several authors have recently postulated that early treatment could lead to a viro-immunological status mimicking that of Elite controllers.27,41-43 Our group has recently described a number of post-treatment controllers who maintained control of viral replication and unchanged CD4+ T cell counts for several years (up to 9.5 years) after discontinuation of long-term cART, which signifies a functional cure.18,44 It is noteworthy that all of these patients started treatment very early after the primary infection. Interestingly, based on our experience, post-treatment controllers share very low and stable reservoirs with HIV controllers and have high CD4+ T cell counts and CD4+/CD8+ ratios that remain stable after cART cessation.43,44 Nevertheless, the frequency of these post-treatment controllers remains undetermined. Moreover, it should be noted that patients with a very low viral reservoir under therapy are not assured of durable PVL control after cART discontinuation, as recently demonstrated by Chun et al.45"
"Viro-immunological responder status
At the LOCF, OVIR status, based on both previously specified outcomes, was achieved by 19/35 PHI patients (54%) and 7/272 (3%) CHI patients (P< 0.0001). At this time, the HIV DNA level (expressed in copy numbers/106 CD4+ T cells) correlated negatively with the CD4+/CD8+ ratio in the PHI group (r = -0.46, P = 0.006), in the CHI group (r = -0.28, P< 0.0001) and in the total patient population (r = -0.43, P< 0.0001).
In logistic regression analysis, cART initiation during PHI and pre-therapeutic HIV DNA level <3.3 log10 copies/106 PBMCs were the only factors predictive of achieving OVIR status (Table 2 and Figure 2d). Within the PHI group, earlier treatment (based on Fiebig stages, nadir CD4+ T cell count or duration from PHI to cART) was not associated with a greater probability of achieving OVIR status.
Applying the same logistic regression analysis to the group of patients treated since CHI (excluding the PHI group), a high CD4+ T cell nadir (>500 cells/mm3) was the only factor predictive of achieving OVIR status (P = 0.03), with an OR of 10.1 (95% CI = 1.7-61.1) (Figure 2e). Within the CHI group, patients who initiated cART before CD4+ fell below 500 cells/mm3 (n = 15) reached the OVIR status in a substantially higher proportion than those treated below 500/mm3 (n = 257) (20% versus 1.5%, respectively, P = 0.004).
Finally, we compared patients treated since CHI in whom cART was started before the CD4+ T cell nadir fell below 500 cells/mm3 (CHI>500, n = 15) with the PHI group (n = 35). This direct comparison was possible since the CHI>500 and PHI patient groups had similar characteristics: duration with PVL <50 copies/mL (median = 2.5 versus 3.1 years; P = 0.7), CD4+ T cell nadir (557 versus 572; P = 0.6) and pre-therapeutic HIV DNA in PBMCs (median = 2.85 versus 3.4 log10 copies/106 PBMCs; P = 0.15). At the LOCF, the CHI>500 and PHI groups achieved similar HIV DNA levels (median = 2.39 versus 2.15 log10 copies/106 PBMCs; P = 0.3), CD4+ T cell counts (median = 915 versus 883 cells/mm3; P = 0.27) and CD4+/CD8+ ratios (median = 1.21 versus 1.31; P = 0.11), but the percentage of CD4+ was significantly different (median = 38% versus 41%; P = 0.03). Overall, CHI>500 patients were less likely than PHI patients to achieve OVIR status (20% versus 54%; P = 0.03) (Figure S3, available as Supplementary data at JAC Online)."
Abstract
Objectives To characterize viro-immunological outcomes following long-term combined antiretroviral therapy (cART) initiated during primary HIV infection (PHI) or chronic HIV infection (CHI) and to identify factors predictive of optimal viro-immunological responder (OVIR) status.
Methods This was a prospective, single-centre cohort study of HIV-1-infected patients on effective cART. Total cell-associated HIV DNA levels and T cell counts before and during treatment were used to identify factors predictive of OVIR status {i.e. low HIV DNA level [<2.3 log10 copies/106 peripheral blood mononuclear cells (PBMCs)], together with normalization of the absolute/relative CD4+ T cell counts and CD4+/CD8+ ratio}.
Results A total of 307 patients were enrolled, of whom 35 started cART during PHI (<4 months post-infection) and 272 during CHI. HIV DNA decay was modelled with a non-linear mixed-effects model that showed two phases of HIV DNA decay, both of which were significantly more pronounced in the PHI group. At the end of follow-up, after a median of 4 years of viral suppression (<50 copies/mL), HIV DNA levels were lower in the PHI group than in the CHI group (median = 2.15 versus 2.84 log10 copies/106 PBMCs; P< 0.0001). Immune reconstitution was more rapid and sustained in the PHI group (median = 883 versus 619 CD4+ cells/mm3; 41% versus 31% CD4+; CD4+/CD8+ 1.31 versus 0.77; all P< 0.0001). Finally, OVIR status was obtained in 19/35 (54%) and 7/272 (3%) patients in the PHI and CHI groups (P< 0.0001), respectively. In a logistic regression analysis, cART initiation during PHI (OR = 16, 95% CI = 3.5-72.3) and HIV DNA level <3.3 log10 before treatment (OR = 4.8, 95% CI = 1.2-19.3) were independently predictive of OVIR status.
Conclusions Initiating cART during PHI represents a major opportunity to reduce HIV reservoirs and achieve optimal immune reconstitution.
Introduction
The effectiveness of combined antiretroviral therapy (cART) is currently based on a plasma HIV-1 RNA viral load (PVL) below the detection limit (<50 copies/mL), together with restoration of CD4+ T cell numbers to >500 cells/mm3.1-3 However, there are several other parameters that also indicate cART success. In untreated patients, the CD4+ percentage and CD4+/CD8+ ratio are markers of disease progression, independent of the absolute CD4+ count.4,5 During effective treatment, the CD4+/CD8+ ratio reflects the T cell activation status, the size of the viral reservoir and the cardiovascular risk.6-8 The size of the viral reservoir, determined by measuring total cell-associated HIV DNA in peripheral blood mononuclear cells (PBMCs), is an independent marker of progression to low CD4+ count and AIDS before treatment.9 The HIV DNA level in PBMCs strongly correlates with HIV DNA levels in gut-associated lymphoid tissues.10 It is also predictive of residual viral load after treatment initiation.11,12 During effective treatment, HIV DNA is still detectable13 and high levels of HIV DNA are predictive of virological failure,14 T cell exhaustion6 and viral rebound after treatment discontinuation.15,16 Contrastingly, a small viral reservoir (<2.3 log10 copies per million PBMCs) is characteristic of Elite controllers and post-treatment controllers, suggesting that sustained control of PVL without treatment is more readily attainable in the case of low reservoirs.17-19
Antiretroviral therapy has significantly improved patient outcomes and reduced HIV-associated illnesses and mortality.20,21 Earlier HIV diagnosis and earlier employment of cART are encouraged;1-3 however, the optimal timing of treatment initiation still remains a matter of debate. The benefit of a long-term treatment initiated early, before the CD4+ T cell count falls below 500 cells/mm3, has not been definitively established. To date, four large, collaborative, observational studies20-23 have attempted to address the issue of when to start treatment: only the NA-ACCORD study recommended initiation of cART before the CD4+ count reaches 500 cells/mm3, but the results of the on-going START study are expected to provide definitive guidance on this issue. Starting cART even earlier, as soon as during the primary phase of infection, has proved to have a positive impact on biological parameters, such as depletion of viral reservoirs/transcription and immune conservation/restoration.24-27 However, the findings of most of these studies are limited by several key points, including a short duration of treatment exposure (no more than 1-4 years) and the lack of direct comparison with patients treated at the chronic phase of infection.
Here we describe the long-term dynamics of blood HIV-1 DNA levels and T cell counts in a large, prospective, single-centre cohort of patients treated successfully for up to a decade, including patients treated within primary infection. We also studied factors predictive of combining a low viral reservoir and optimal immune reconstitution under therapy.
Discussion
This study is, to our knowledge, the first to report the very long-term (≥10 years) impact of continuous cART on viral reservoirs and immune reconstitution in a large prospective cohort, comparing patients treated since PHI and CHI. It emphasizes the large viro-immunological benefit of initiating treatment at the PHI phase; 54% of patients who started treatment during PHI achieved both a very low blood viral reservoir (HIV DNA level <2.3 log10 copies/106 PBMCs) and normal values of all three immunological parameters (CD4+ count and percentage, and CD4+/CD8+ ratio). In contrast, only 3% of patients achieved these results when cART was started later, i.e. during the chronic phase, and interestingly, these patients were characterized by a high CD4+ nadir (>500 cells/mm3). Nevertheless, such benefits should be balanced with the risk and cost of a longer exposure to antiretroviral drugs.24
The range of HIV DNA levels at different stages of infection is smaller than the range of HIV RNA levels,13 thus the difference in HIV DNA levels we observed between PHI and CHI groups after several years on effective cART is impressive. The technique used permits quantification of all forms of cellular HIV DNA: unintegrated linear, 1LTR and 2LTR circular forms, as well as integrated forms. The vast majority of total HIV DNA levels were obtained in patients with undetectable PVL, thus we are confident that they reflect integrated HIV DNA.6,32 Recently Ananworanich et al.33 demonstrated that HIV DNA levels increase gradually during the first weeks after acute infection, suggesting that the earlier cART is introduced, the lower the HIV DNA level is to be decreased. This observation was supported by Archin et al.,34 who showed that cART reduced the generation of latently infected CD4+ T cells in patients treated during acute seronegative HIV-1 infection. Nevertheless, in our study only 10% of patients treated at PHI were seronegative (i.e. Fiebig I or II). Additionally, the PHI and CHI groups shared similar HIV DNA levels before treatment initiation. Thus we hypothesize the final difference between our groups is more likely due to the decay during cART, although we cannot exclude both mechanisms (restriction and depletion) combined.
The mathematical modelling of HIV DNA decay emphasizes the different dynamics of PHI and CHI groups, even if both responded to a general biphasic curve model. This finding is concordant with previous reports,35-37 but the longer duration of cART and the comparison of PHI and CHI groups in our study gives new insight to this model. Indeed, we enrolled both patients who were about to start treatment and those who had been on treatment for several years (up to 14 years) in order to model the initial decrease as well as long-term decay. Since patients received cART for various periods, the model took into account the fact that the HIV DNA level was quantified at different times in the various patients.31 In the PHI group there is a fast initial decay after cART initiation, with a half-life of 113 days, similar to those previously reported (210 and 116 days for Strain et al.36 and von Wyl et al.,37 respectively). In contrast, we found a slow decay in the second phase for up to a decade of successful therapy; such a finding was reported by Strain et al.36 in a few patients (n = 8) treated up to 200 weeks, but was not observed by von Wyl et al.37 in a larger study (n = 59). The latter study evoked a decay rate no longer significantly different from zero, probably because their analysis employed a shorter follow-up (48 months) than our study. The slow HIV DNA decay we found during the second phase of follow-up was significantly stronger in patients treated since PHI than in those treated since CHI. In patients treated since CHI, Viard et al.35 noted a plateau phase between 3 and 5 years of effective therapy, but as with the publication from von Wyl et al.,37 this conclusion could be due to a shorter observation time on treatment. Altogether, these differences in HIV DNA decay between the PHI and CHI groups strongly suggest that early treatment prevents and/or decreases the establishment of large viral reservoirs in resting cells with long half-lives, while cART has a very low impact on infected resting cells that are well stabilized at the chronic phase. Additionally, cART initiated during PHI could preserve immune functions (innate and adaptive), enabling better immune reconstitution and hence more efficient clearance of the pool of infected cells, even during the second (slow) phase of HIV DNA decay.
Several studies of patients who started treatment in the chronic phase suggest that earlier cART is always associated with better CD4+ T cell recovery, even after 10 years of viral suppression.38-40 Although the number of CD4+ T cells appears to increase gradually throughout the first decade of viral suppression, it is remarkable that the initial stratification based on the pre-treatment nadir remained unchanged until the end of these studies.39,40 We believe that our study is the first to describe the evolution of T cell counts after 10 years of continuous viral suppression (without blips >200 copies/mL) initiated as soon as PHI. While we observed long-term advantages to being treated since PHI (versus CHI) for all immunological parameters taken separately, the comparison was even more striking when we looked at the combination of all three markers of immune reconstitution. Like Kelley et al.,38 we not only found that some CHI patients never achieved CD4+ T cell counts above the threshold of 500 cells/mm3, but we also showed that the majority of them never achieved normal T cell counts-particularly a normal CD4+/CD8+ ratio-even after 14 years of antiretroviral therapy. In contrast, 90% of the group treated since PHI was able to reach that goal after 6 years of therapy, and almost all within a decade of therapy.
The potential benefits of combining sustained control of viral replication, a small viral reservoir and an optimal immune reconstitution are highlighted by studies of rare natural (Elite) or post-treatment controllers. Several authors have recently postulated that early treatment could lead to a viro-immunological status mimicking that of Elite controllers.27,41-43 Our group has recently described a number of post-treatment controllers who maintained control of viral replication and unchanged CD4+ T cell counts for several years (up to 9.5 years) after discontinuation of long-term cART, which signifies a functional cure.18,44 It is noteworthy that all of these patients started treatment very early after the primary infection. Interestingly, based on our experience, post-treatment controllers share very low and stable reservoirs with HIV controllers and have high CD4+ T cell counts and CD4+/CD8+ ratios that remain stable after cART cessation.43,44 Nevertheless, the frequency of these post-treatment controllers remains undetermined. Moreover, it should be noted that patients with a very low viral reservoir under therapy are not assured of durable PVL control after cART discontinuation, as recently demonstrated by Chun et al.45
A limitation of our study is its observational nature; however, despite treatment strategies not being randomized, they were concordant with national guidelines in all cases. Systematic differences between the groups of individuals treated as soon as during PHI and those treated in CHI may have affected our analysis, such as the severity of the acute retroviral syndrome. Nevertheless, patients in the PHI group had heavily symptomatic primary infection, a feature associated with less-favourable immuno-virological parameters and disease outcomes.46 Finally, this reinforces the benefit of long-term cART initiated during primary HIV-1 infection, as it achieved the better observed immune reconstitution and depletion of HIV reservoirs, despite unfavourable immuno-virological parameters before treatment.
In conclusion, we show that primary HIV infection represents a critical period (possibly a unique opportunity) during which cART can induce a profound decrease in the viral reservoir together with rapid optimal immune restoration. In this setting, long-term treatment provides the most favourable chance of obtaining the lowest level of residual viral reservoir and better immune reconstitution. Regarding patients with a later diagnosis of HIV infection, we show that cART initiation before the CD4+ T cell count falls below 500 cells/mm3 may still induce OVIR status, whereas delaying treatment greatly reduces the chances of reaching this status, even after up to 14 years of sustained viral suppression. Our findings also support public health programmes designed to promote HIV testing and treatment ('test and treat'), especially among highly exposed populations, as early treatment may not only limit the risk of transmission,47 but may also benefit the patients themselves.
Patients and methods
Study population and data collection
From January 2005 to January 2011, all HIV-1-infected patients attending Orleans Regional Hospital that were receiving and responding to cART (i.e. PVL value <50 copies/mL) were prospectively included in this observational cohort study (main study), with baseline defined as the date of the first PVL value <50 copies/mL following cART initiation. In addition, untreated patients eligible for cART according to national guidelines were screened in an 'induction' substudy and were enrolled in the main study as soon as their PVL became undetectable (<50 copies/mL) on cART (Figure S1, available as Supplementary data at JAC Online).
Primary HIV infection (PHI) was defined by a negative or weakly positive ELISA with incomplete HIV-1 western blot (≤4 bands) and positive PVL and/or a positive HIV ELISA following a negative ELISA within the preceding 3 months. Patients who started cART within 4 months after this diagnosis of PHI were compared with patients who started cART later, i.e. during the chronic phase [chronic HIV infection (CHI) group]. Treatments offered to patients from both groups were in accordance with national guidelines.28
The patients' medical and antiretroviral treatment histories were collected, together with the T cell nadir and PVL before treatment. Results of physical examinations, relevant events, antiretroviral treatment changes and routine laboratory tests (CD4+ and CD8+ T cell counts, and PVL) were collected prospectively, three to four times a year.
HIV DNA levels were measured in PBMCs at least once a year for as long as PVL remained undetectable and the data were collected prospectively. In the induction substudy, HIV DNA levels were measured at least once before treatment initiation, then twice during the first year on therapy and once a year thereafter. Patients remained in the study until viral failure (VF) occurred (i.e. HIV-RNA >50 copies/mL and <200 copies/mL in two consecutive samples or >200 copies/mL in one sample), or until they were lost to follow-up; all subsequent data were censored for analysis.
The protocol was conducted in accordance with the Declaration of Helsinki and was approved by the local ethics committee (Tours-Ouest-1). All participants gave their written informed consent.
Plasma HIV-RNA levels
PVL was quantified with a real-time HIV-1 PCR assay (Abbott Molecular Inc., Des Plaines, IL, USA) as recommended by the manufacturer. The detection limit was initially 50 HIV-RNA copies/mL, then 20 copies/mL. Low-level viraemia was defined as values between 20 and 50 copies/mL. 'Blips' were not considered to represent VF if they remained below 200 copies/mL and if the values obtained immediately before and after were below 50 copies/mL.
Measurement of total cell-associated HIV DNA in PBMCs
The HIV DNA level in PBMCs was quantified in whole blood using the Agence Nationale de Recherches sur le Sida et les Hepatites Virales (ANRS) real-time PCR method (Biocentric, Bandol, France), as previously described.29 The detection limit is 5 copies/PCR. All samples were tested in real time from January 2005 at the Necker Hospital virology laboratory with the same assay throughout the study. Total cell DNA extracts were systematically tested in duplicate and retested in quadruplicate in case of very low levels. Results were calculated using the blood formula and expressed in copy numbers per million PBMCs (for the main analysis), copy numbers per million CD4+ T cells and copy numbers per millilitre of blood.30
HLA class I phenotyping
HLA-B two-digit phenotypes were determined with sequence-specific oligonucleotide primed PCR or sequence-specific primer PCR.
Outcomes
To assess the proportion of patients achieving the status of 'optimal viro-immunological responder' (OVIR), defined as a low viral reservoir (HIV DNA <2.3 log10 copies/106 PBMCs), together with the normalization of markers of immune reconstitution (absolute CD4+ T cell counts >500 cells/mm3 and percentage of CD4+ T cells >30% and CD4+/CD8+ ratio >1) up to the last observation carried forward (LOCF). The threshold used for the viral reservoir was based on the ANRS 116 SALTO study, which demonstrated that a low HIV DNA level in PBMCs (<2.3 log10 copies/106 PBMCs) at antiretroviral treatment interruption predicted a longer time to treatment resumption, independently of the CD4+ nadir.16 HIV DNA decay was modelled according to the time cART was started (primary or chronic infection).
Statistical analyses
Statistical analyses were performed to describe HIV DNA decay using the R package (www.r-project.org) with the NLME library (R Foundation for Statistical Computing, Vienna, Austria). We used several approaches, including linear and non-linear models.31 Analyses were performed on log10 transformed data. The population parameters were estimated in a maximum-likelihood function formulation in order to rank the capacity of the different models to represent the patients' data. Individual CD4+ T cell slopes were also modelled to avoid fluctuations around the values of interest.
Categorical data were compared with Fisher's exact test or the X2 test, and continuous variables with the Kruskal-Wallis test. Associations between outcomes of interest and various factors, including all baseline characteristics, were tested by multivariate logistic regression analysis. Kaplan-Meier P values were based on the log-rank test. Linear correlations were analysed by using Spearman's test.
Results
Characteristics of the cohort
A total of 307 patients fulfilled the inclusion criteria of the main study: 35 started cART during PHI (at Fiebig stages II, III, IV and V in 10%, 10%, 26% and 54%, respectively) and 272 started cART during CHI. All patients in the PHI group were symptomatic at the primary infection. The main characteristics of the patients are summarized in Table 1. Age was similar in the two groups, as were the distribution of HIV risk groups, ethnicity and HLA-B alleles. Patients in the PHI group were more likely to be male, symptomatic during PHI and uninfected by hepatitis viruses, while those in the CHI group had a lower CD4+ nadir and a more frequent history of CDC stage B or C HIV-related events. At the LOCF, patients in the PHI group were more likely (P< 0.0001) to withdraw from the study without VF, owing to requests for treatment interruption, as was considered acceptable according to French national guidelines until 2008. Overall, the proportion of PVL assays showing blips was 3.4%, and 26% of patients had low viraemia (20< PVL< 50 copies/mL) at the LOCF, with no significant difference between the PHI and CHI groups (P = 0.22 and P = 0.21, respectively).
Of the 73 patients (27% of the cohort) enrolled in the induction substudy, 22 started cART during PHI and 51 during CHI. When stratified for the timing of cART initiation (PHI versus CHI), the patients in this substudy had very similar demographics, baseline viro-immunological characteristics and treatment histories to the patients enrolled directly in the main study (data not shown). At the end of the main study, patients included through the substudy had similar outcomes (viral control, VF or withdrawal without VF) to the rest of the cohort, except for shorter exposure to cART and a shorter period with undetectable PVL (P< 0.0001).
Decay of PBMC HIV DNA levels
During the 6 year study period, 1103 HIV DNA tests were performed among the 307 participants. In the whole cohort, the median number of HIV DNA tests per patient was 3 (IQR = 2-5) and the median interval between the first and last test was 2.3 years (IQR = 1.1-3.5). The median number of tests was six in the PHI group and three in the CHI group. In the substudy population, HIV DNA levels before therapy were similar in the two groups (Table 1).
The HIV DNA data fitted most appropriately to a bi-exponential mixed-effects model; this model took into account the fact that patients contributed several data points and at various times relative to the initiation of cART. Random effects accounted for individual differences from the population average described by the fixed effect. Decay rates of HIV DNA levels during treatment, V(t), were modelled as a two-phase decay according to the following equation:
V(t) = A1 X exp[-exp(lrc1) X t] + A2 X exp[-exp(lrc2) X t],
where A1 and A2 represent the intercepts (size of two different reservoirs with rapid and slow clearance, respectively), exp(lrc1) and exp(lrc2) are the first- and second-phase decay rates and t is the duration of undetectable PVL. Time t = 0 corresponded to the first sample with HIV RNA <50 copies/mL. Figure 1 represents all HIV DNA levels over time, together with modelled decay in the PHI and CHI groups. During the first 2 years, fast initial decay of HIV DNA levels was observed in patients treated since PHI and in those treated since CHI, and with similar half-lives [113 days (95% CI = 84-157) and 146 days (95% CI = 96-219), respectively]. The second phase of decay was much slower, and the half-life was significantly shorter in the PHI group (25 years, 95% CI = 15-43) than in the CHI group (377 years, 95% CI = 22-6552; P< 0.001).
At the LOCF, PHI patients had much lower final HIV DNA levels (median = 2.15 log10 copies/106 PBMCs, IQR = 1.7-2.47) than CHI patients (median = 2.84 log10 copies/106 PBMCs, IQR = 2.52-3.09; P< 0.0001). HIV DNA levels were <2.3 log10 copies/106 PBMCs in 69% (24/35) of PHI patients and 13% (35/272) of CHI patients (P< 0.0001). Final HIV DNA levels were also much lower in PHI patients when expressed in copy numbers per million CD4+ T cells (median = 2.66, IQR = 2.39-2.95) and copy numbers per millilitre of blood (median = 2.7, IQR = 2.17-2.92) than in CHI patients (median = 3.41, IQR = 3.1-3.71; P< 0.0001 and median = 3.19, IQR = 2.87-3.46; P< 0.0001, respectively).
In the substudy population, the median period with undetectable PVL was 1.5 years (IQR = 0.7-2.7) in both the PHI and CHI groups; the median change in HIV DNA levels from pre-treatment to the LOCF was -1.21 log10 copies/106 PBMCs (IQR = -0.94 to -1.64) in the PHI group and -0.51 (IQR = -0.24 to -0.90) in the CHI group (P< 0.0001).
In logistic regression analysis, cART initiation during PHI, a high CD4+ nadir (>500 cells/mm3) and a pre-therapeutic HIV DNA level <3.3 log10 copies/106 PBMCs were predictive of reaching a low PBMC reservoir (<2.3 log/106 PBMCs) during therapy (Table 2 and Figure 2a).
Immune reconstitution
Both PHI and CHI groups had similar increases in the three immunological markers. Nevertheless, final values at the LOCF differed markedly, always in favour of the PHI group: the median CD4+ T cell count was 883 cells/mm3 versus 619 cells/mm3 (P< 0.0001); the median CD4+ T cell percentage was 41% versus 31% (P< 0.0001); and the median CD4+/CD8+ ratio was 1.31 versus 0.77 (P< 0.0001). All three markers normalized in 28/35 PHI patients (80%) and 72/272 (26%) CHI patients (P< 0.0001). The CD4+ T cell count was above the threshold of 500 cells/mm3 in 34/35 (97%) PHI patients and 191/272 (70%) CHI patients (P = 0.0002). Regardless of the duration of undetectable PVL, the PHI group always showed better T cell reconstitution on cART than the CHI group (Figure S2, available as Supplementary data at JAC Online).
In logistic regression analysis, treatment since PHI, a high nadir CD4+/CD8+ ratio (>0.5) before cART initiation and a long duration of undetectable PVL (>4 years) were predictive of optimal immune restoration, whereas a low nadir CD4+ T cell count (<200 cells/mm3) was associated with a lower chance of optimal immune restoration (Table 2 and Figure 2b and c).
Viro-immunological responder status
At the LOCF, OVIR status, based on both previously specified outcomes, was achieved by 19/35 PHI patients (54%) and 7/272 (3%) CHI patients (P< 0.0001). At this time, the HIV DNA level (expressed in copy numbers/106 CD4+ T cells) correlated negatively with the CD4+/CD8+ ratio in the PHI group (r = -0.46, P = 0.006), in the CHI group (r = -0.28, P< 0.0001) and in the total patient population (r = -0.43, P< 0.0001).
In logistic regression analysis, cART initiation during PHI and pre-therapeutic HIV DNA level <3.3 log10 copies/106 PBMCs were the only factors predictive of achieving OVIR status (Table 2 and Figure 2d). Within the PHI group, earlier treatment (based on Fiebig stages, nadir CD4+ T cell count or duration from PHI to cART) was not associated with a greater probability of achieving OVIR status.
Applying the same logistic regression analysis to the group of patients treated since CHI (excluding the PHI group), a high CD4+ T cell nadir (>500 cells/mm3) was the only factor predictive of achieving OVIR status (P = 0.03), with an OR of 10.1 (95% CI = 1.7-61.1) (Figure 2e). Within the CHI group, patients who initiated cART before CD4+ fell below 500 cells/mm3 (n = 15) reached the OVIR status in a substantially higher proportion than those treated below 500/mm3 (n = 257) (20% versus 1.5%, respectively, P = 0.004).
Finally, we compared patients treated since CHI in whom cART was started before the CD4+ T cell nadir fell below 500 cells/mm3 (CHI>500, n = 15) with the PHI group (n = 35). This direct comparison was possible since the CHI>500 and PHI patient groups had similar characteristics: duration with PVL <50 copies/mL (median = 2.5 versus 3.1 years; P = 0.7), CD4+ T cell nadir (557 versus 572; P = 0.6) and pre-therapeutic HIV DNA in PBMCs (median = 2.85 versus 3.4 log10 copies/106 PBMCs; P = 0.15). At the LOCF, the CHI>500 and PHI groups achieved similar HIV DNA levels (median = 2.39 versus 2.15 log10 copies/106 PBMCs; P = 0.3), CD4+ T cell counts (median = 915 versus 883 cells/mm3; P = 0.27) and CD4+/CD8+ ratios (median = 1.21 versus 1.31; P = 0.11), but the percentage of CD4+ was significantly different (median = 38% versus 41%; P = 0.03). Overall, CHI>500 patients were less likely than PHI patients to achieve OVIR status (20% versus 54%; P = 0.03) (Figure S3, available as Supplementary data at JAC Online).
| |
| |
| |
|
|
|