| |
Amount of HIV DNA in Peripheral Blood Mononuclear Cells is Proportional to the Severity of HIV-1-Associated Neurocognitive Disorders
|
| |
| |
Download the PDF here
J Neuropsychiatry Clin Neurosci. December 01, 2009
Bruce Shiramizu, M.D.
Andrew E. Williams, Ph.D.
Cecilia Shikuma, M.D.
Victor Valcour, M.D.
"For each unit increase in log of HIV DNA, there is a decrease in neuropsychiatric deficit. Neuropsychological deficits were significantly associated with entry HIV DNA in all deficits (Table 2) (regression coefficients range from -0.24 to -0.07, p<0.05). The effect of HIV DNA on neuropsychiatric deficits remained significant after adjusting for age, ethnicity, and estimated premorbid IQ."
Abstract
Human immunodeficiency virus (HIV) DNA in peripheral blood mononuclear cells was previously associated with neuropsychological function. By including individuals encompassing the full range of HIV-1-associated neurocognitive disorders, this study reports results from subjects with normal cognition, minor cognitive motor disorder, and HIV-1-associated dementia. Individuals with normal cognition had relatively low HIV DNA levels compared to those with minor cognitive motor disorder and HIV-1-associated dementia. Neuropsychological deficits were significantly associated with entry HIV DNA in all domains. These findings demonstrate for the first time that the severity of HIV-1-associated neurocognitive disorders is proportional to the amount of circulating HIV DNA.
For each unit increase in log of HIV DNA, there is a decrease in neuropsychiatric deficit. Neuropsychological deficits were significantly associated with entry HIV DNA in all deficits (Table 2) (regression coefficients range from -0.24 to -0.07, p<0.05). The effect of HIV DNA on neuropsychiatric deficits remained significant after adjusting for age, ethnicity, and estimated premorbid IQ.
Methods
While great progress has been made in understanding the pathogenesis of HIV-1-associated dementia, the complete picture of the mechanisms involved remains unclear. Because the neuropathogenesis of HIV-1-associated neurocognitive disorders is not completely known, identifying unique markers for HIV-1-associated neurocognitive disorders has been challenging.1 In the current era of highly active antiretroviral therapy, neurocognitive impairment appears to persist and if biomarkers for HIV-1-associated neurocognitive disorders could be identified, this may provide clues to define mechanisms involved in disease progression.1-6 Factors such as activated macrophages and CSF HIV RNA levels were previously found to be potential markers for neurocognitive decline and need further studies to be validated.1,7-9 In patients on highly active antiretroviral therapy, CSF RNA levels may be elevated in those with worsening neurocognitive status, but the specific role that the virus plays in neuropathogenesis is still not entirely clear.10,11 Plasma HIV RNA levels may or may not correlate with neurocognitive decline, and thus circulating viral RNA might be interacting with other factors in causing HIV-1-associated neurocognitive disorders.7,9,12-17
Our group and others have previously reported HIV DNA levels to be potentially important in HIV disease prognosis, including the diagnosis of HIV-1-associated dementia.18-22 One rationale for pursuing HIV DNA further as a marker for HIV-1-associated dementia is that currently available highly active antiretroviral therapy regimens have little effect on HIV DNA, allowing for persistence of viral DNA in reservoirs.23-25 We previously demonstrated high HIV DNA copies in peripheral blood mononuclear cells from individuals with and those without HIV-1-associated dementia from two different cohorts from Hawaii (n=49) and Thailand (n=30).22,26 In the study with the Hawaii Aging with HIV Cohort, we further demonstrated that specific neuropsychological deficits were associated with HIV DNA levels.27 However both the studies from Hawaii and Thailand were limited to individuals diagnosed with HIV-1-associated dementia and those with normal cognition, and did not include subjects with milder forms of impairment (i.e., minor cognitive motor disorder) which potentially includes a significant proportion of HIV-1-infected individuals with associated neurocognitive disorders.28-30 The purpose of the current study was to assess HIV DNA in peripheral blood mononuclear cells from a separate subset of patients from the Hawaii Aging with HIV Cohort (n=189) not previously analyzed, and to determine if patients with minor cognitive motor disorder demonstrate a continuum increase in peripheral blood mononuclear cell HIV DNA compared to those with HIV-1-associated dementia and those with normal cognition. Additionally, we hypothesized that HIV DNA would be associated with individual neurocognitive domains, as we previously reported in a small subset from the Hawaii Aging with HIV Cohort.27 If positive association were found, then peripheral blood mononuclear cell HIV DNA may prove to be an important factor in the pathogenesis of neurocognitive dysfunction.
The Hawaii Aging with HIV Cohort
The Hawaii Aging with HIV Cohort is a longitudinal cohort established to examine HIV-1-associated neurocognitive disorders in older (≥50 years old) and younger (20-39 years old) HIV-seropositive individuals. The Hawaii Aging with HIV Cohort is unique with less than 5% of the participants identified as intravenous drug users, which is lower than other cohorts.31-35 Following informed consent guidelines established by the University of Hawaii Institutional Review Board, participants living in Hawaii were enrolled; we excluded those with a major psychiatric or neurologic disorder, a history of head injury with loss of consciousness greater than 1 hour, current or past opportunistic infection with brain involvement, a diagnosed learning disability, or delirium due to medications at the time of examination. For the current study, subjects who were included in the previous study were also excluded from this analysis.36 Subjects were evaluated at entry into the cohort and yearly for 4 years. Subsequent follow-up visits were scheduled in advance at the patient's convenience and ability to complete the neurocognitive testing in one visit in the absence of any acute medical crisis.
Participant evaluations included demographic information, medical history, neurologic examination including the United Parkinson's Disease Rating Scale to examine for extrapyramidal signs, medication/adherence history, DSM-IV-based substance abuse/dependence inventory, immunologic and virologic laboratory tests, and neuropsychiatric testing; as previously reported.27 The 80-minute neuropsychiatric test battery, adapted from the NorthEast AIDS Dementia Cohort, assessed multiple cognitive domains affected by HIV-1 and included the following: choice and sequential reaction time from the California Computerized Assessment Package, Rey Auditory Verbal Learning Test (RAVLT), Rey Osterreith Complex Figure (RCF) Copy and Recall, Trail Making tests A and B, WAIS-R Digit Symbol, Grooved Pegboard (dominant and nondominant hands), Verbal fluency test, Animal Naming, Boston Naming Test, the WAIS-R Digit Span (forward and backward), and Timed Gait. Depression symptomatology was assessed using the Beck Depression Inventory (BDI).37 Normative neuropsychiatric data for individuals with a high school or greater education were derived from the Multicenter AIDS Cohort Study consisting of 733 HIV-1-seronegative subjects with risk profiles similar to the Hawaii cohort. For individuals with less than a high school education, normative neuropsychological data from the AIDS Link to IV Experience study (n=150) was used.38 These two normative sets have few individuals over 54 years old. Thus, for individuals over 54, alternative published normative data were used.39,40 Normative data for the Rey Osterreith Complex Figure were taken from alternative published norms for individuals over 59 and the main Multicenter AIDS Cohort Study normative set for individuals under 60 years old.41 A similar battery with a large overlap in norms was shown to be appropriate for HIV-1-infected individuals of similar ethnic diversity.42 Various normative data were required due to the use of a comprehensive test battery and the inclusion of both younger and older subjects. The normative data were selected as the best possible dataset for this population with a long history of applications in HIV-1 research (e.g., Multicenter AIDS Cohort Study). Application of these norms was guided by the clinical neuropsychologists on the team. All test results were transformed to Z scores using appropriate age and education-matched normative data sets. Scores for cognitive domains (motor skills/motor speed; verbal memory; visual memory; working memory, attention, and concentration; learning; recognition memory; visuospatial abilities; executive functioning; language) were calculated by averaging the Z scores of the neuropsychiatric tests corresponding to the domains they were intended to measure, as previously published.27 All patients who had research-based neurocognitive diagnoses using the American Academy of Neurology 1991 criteria (normal cognition, minor cognitive motor disorder, and HIV-1-associated dementia) without confounds (methamphetamine/cocaine use, stroke/transient ischemic attacks) who consented and donated blood for research studies were included in the analyses.43
Specimens and Assessment of Peripheral Blood Mononuclear Cell HIV DNA Copies
Specimens were obtained and stored at the time of study entry. Plasma VL (Amplicor HIV-1 Monitor Ultra Sensitive Test, Roche Diagnostics, Switzerland) and CD4 cell counts were performed by a certified clinical laboratory. Genomic DNA was extracted from peripheral blood mononuclear cells as per manufacturer's protocol (Qiagen, Valencia, Calif.). The quantity and quality of the DNA was assessed by UV spectrophotometry (DNA concentration and OD 260/280 nm absorbance ratio) and polymerase chain reaction (PCR) as previously described.44 The assay for measuring HIV DNA, which was previously published, was used to calculate the number of copies of HIV-1 DNA per 106 cells.22,26,27
Statistical Analyses
The statistical analyses were performed without division into the age groups of the parent study. All tests of hypotheses were used for participants classified as normal cognition, minor cognitive motor disorder, and HIV-1-associated dementia. The primary analysis was performed on specimens obtained at the earliest time of participation in which matching clinical laboratory data and neurocognitive status were available. The Mann-Whitney Rank Sum Test and one-way analysis of variance (ANOVA) were used to test for differences between groups. We examined the association between HIV DNA and each of the nine neuropsychiatric domains in a series of multilevel longitudinal regression models. The level one variable in the model included initial neuropsychiatric deficits and annual rates of change over 5 years. All analyses were conducted using SAS® v9.1 PROC MIXED specifying maximum likelihood as the estimation method and an unstructured covariance matrix. We examined the association between HIV DNA and baseline neuropsychiatric deficit and annual rate of change of each deficit, as indicated by the TYPE III fixed effect for HIV DNA after it was added to the unconditional growth model. To determine whether the obtained estimates were attributable to other patient characteristics we added age, ethnicity, CD4 cell count, and premorbid IQ.
Results
The demographics of the 189 subjects from the Hawaii Aging with HIV Cohort are similar to what is reported in the state of Hawaii. The cohort, as initially designed, enrolled older (≥50 years old) and younger (20-39 years old) subjects; thus the mean age was 44 years old, SD=11 years (range=21-73 years). The mean years of education was 13.93 years (SD=2.25 years) with 51% Caucasian and 37% Asian-Pacific Islander; 15% were women and 85% were men.32 The HIV RNA levels (VL) and CD4 cell counts among the three groups (normal cognition, minor cognitive motor disorder, and HIV-1-associated dementia) were not statistically significant (p=0.78 and p=0.36, respectively) (Table 1).
The analysis comparing HIV DNA in individuals with normal cognition (NC), minor cognitive motor disorder (MCMD) and HIV-1-associated dementia (HAD) showed a significant effect among all three groups (p<0.001; NC=83, MCMD=85, HAD=21; Table 1). To examine whether the HIV DNA association was due to HIV RNA levels, we repeated the analysis among participants with undetectable plasma HIV RNA viral load (N=95) and found that the significance was upheld in those with HIV-1-associated dementia (n=11) relative to those with minor cognitive motor disorder (n=40) and normal cognition (n=44) (p<0.001, Table 1); with similar CD4 cell counts (p=0.39). Similarly, in subjects with detectable plasma HIV RNA viral load (n=94), HIV DNA was also higher in those with HIV-1-associated dementia (n=10) relative to those with minor cognitive motor disorder (n=45) and normal cognition (n=39) (p<0.001, Table 1). The results from this relatively large separate cohort from Hawaii Aging with HIV Cohort showed a positive correlation; therefore the data were reanalyzed to include the smaller subset from Hawaii Aging with HIV Cohort previously reported. The combined data, using a univariate logistic regression model with a generalized logits link function, demonstrated a strong probability of cognitive diagnosis (normal cognition, minor cognitive motor disorder, or HIV-1-associated dementia) with HIV DNA (Figure 1 ).
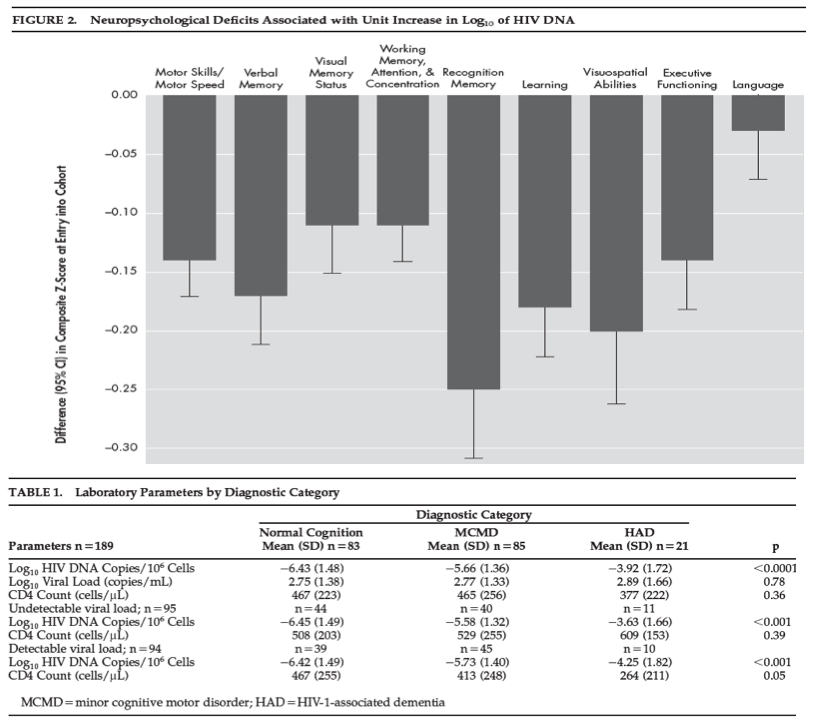
The decrement in baseline of each neuropsychiatric deficit which is associated with HIV DNA is plotted in Figure 2 , adjusted for age, ethnicity, CD4 cell count, and estimated premorbid IQ, where ethnicity and IQ are not confounded with cognitive diagnosis. For each unit increase in log of HIV DNA, there is a decrease in neuropsychiatric deficit. Neuropsychological deficits were significantly associated with entry HIV DNA in all deficits (Table 2) (regression coefficients range from -0.24 to -0.07, p<0.05). The effect of HIV DNA on neuropsychiatric deficits remained significant after adjusting for age, ethnicity, and estimated premorbid IQ.
| |
| |
| |
|
|
|