| |
Two studies address diabetes risks with statins-one good news, one so-so
|
| |
| |
Download the PDF here
Download the PDF here
May 23 2013
Theheart.org
Toronto, ON - The debate about the potential risks of new-onset diabetes in statin-treated patients is addressed in two separate studies published by Canadian researchers looking at patients treated with the LDL-lowering drugs [1,2]. While one study is reassuring for older patients treated with statins, with researchers finding no evidence of an increased risk of diabetes in acute coronary syndrome (ACS) patients, the other suggests that the more potent statins, such as rosuvastatin (Crestor, AstraZeneca), atorvastatin, and simvastatin, do pose an increased risk compared with pravastatin.
In a study published online May 14, 2013 in Circulation: Cardiovascular Quality and Outcomes, Dr Dennis Ko (Sunnybrook Health Sciences Center, Toronto, ON) and colleagues explain that while clinical-practice guidelines for ACS patients advocate for the use of intensive-dose statin therapy, the data suggesting an increased risk of diabetes with the drugs have raised some concerns.
The group analyzed data of more than 17 000 patients 65 years of age and older hospitalized with MI in Ontario, Canada, between 2004 and 2010. Just over 52% of the ACS patients were treated with intensive statin therapy, defined as atorvastatin >40 mg, rosuvastatin >20, and simvastatin >60 mg. Atorvastatin <40 mg, rosuvastatin <20 mg, simvastatin <60 mg, and any dose of fluvastatin, lovastatin, and pravastatin, were considered moderate-dose statin therapy and prescribed to 48% of the treated patients.
In total, 8540 matched pairs who had a similar likelihood of receiving intensive-dose or moderate-dose statin therapy were included in the analysis.
Five years after hospitalization for ACS, 13.6% of patients who received intensive statin therapy had a new diagnosis of diabetes. Comparatively, 13.0% of patients prescribed moderate-dose therapy had a new diagnosis of diabetes.
The difference was not statistically significant. Intensive-dose statin therapy did result in a significantly lower risk of repeat hospitalizations for ACS or death compared with moderate-dose therapy (44.8% vs 46.5%, respectively; p=0.04). The reduction in ACS alone was also statistically significant (22.2% vs 23.5%; p=0.04), while differences in mortality risk at five years were not.
In a statement, Ko said the study "provides evidence that shows intensive statins are underutilized in older patients," and the data should reassure physicians and encourage them to treat more patients with high-dose statin therapy.
FDA mandates label change in 2012
In 2012, as reported by heartwire, the US Food and Drug Administration mandated a labeling change to the entire drug class, issuing a warning that statins can raise blood sugar and HbA1c levels (pravastatin was exempted from the label change). The risk of diabetes began to gain traction first with the Justification for the Use of Statins in Primary Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) study, where investigators reported a 27% increase in the risk of diabetes in patients taking rosuvastatin compared with placebo.
Other analyses and meta-analyses confirmed the increased risk with statins in JUPITER, with an analysis from the Women's Health Initiative (WHI) showing a 48% increased risk of diabetes among women, while an analysis of PROVE-IT, A to Z, TNT, IDEAL, and SEARCH showed that high-dose statin therapy increased the risk of diabetes by 12%. In addition, a similar meta-analysis found that statin therapy increased the risk of diabetes by 9%.
Higher risks compared with pravastatin
While Ko et al suggest it safe to treat older patients with intensive statin therapy, another study, this one published May 23, 2013 in BMJ, does find that patients are at an increased risk of new-onset diabetes when treated with rosuvastatin, atorvastatin, and simvastatin compared with those treated with pravastatin.
Led by Dr Aleesa Carter (Toronto General Hospital, ON), the researchers explain that despite concerns about the risk of new-onset diabetes among statin-treated patients, pravastatin is exempt from the FDA label change. In fact, pravastatin was associated with a 30% lower risk of diabetes compared with placebo-treated patients in the West of Scotland Coronary Prevention Study (WOSCOPS).
Over a 14-year period, the researchers identified from provincial databases 471 250 patients with no history of diabetes treated with a statin. Of these, just under half were treated with a statin for primary prevention, while 51.7% received a statin for secondary prevention. The patients were older-median age at the start of treatment was 73 years-and more than half were women.
Atorvastatin was the most commonly prescribed medication, accounting for half of all new prescriptions, followed by rosuvastatin, simvastatin, pravastatin, lovastatin, and fluvastatin.
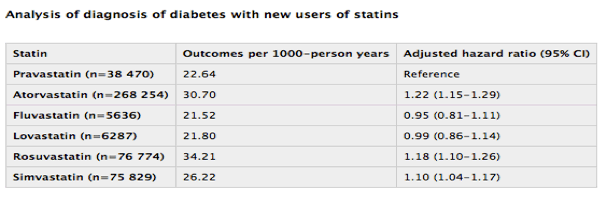
Compared with pravastatin, atorvastatin, rosuvastatin, and simvastatin were associated with a 22%, 18%, and 10% increased risk of new-onset diabetes, respectively. There was no risk observed with fluvastatin or lovastatin. In a subgroup analyses, the results were consistent in the primary- and secondary-prevention cohorts.
There was also an increased risk of new-onset diabetes among those treated with moderate-dose statins (hazard ratio 1.22; 95% CI 1.19-1.26) and high-dose statins (HR 1.30; 95% 1.20-1.40) compared with low-dose therapy. The researchers note that the risk associated with rosuvastatin appears to be dose related and dependent on the duration of treatment. "The increased risk with rosuvastatin significantly decreased after covariate adjustment and became nonsignificant once dose was taken into consideration," write Carter et al.
Sources
1. Ko DT, Wijeysundera HC, Jackevicius CA, et al. Diabetes and cardiovascular events in older myocardial infarction patients prescribed intensive-dose and moderate-dose statins. Circ Cardiovasc Qual Outcomes 2013; 6: 315-22. DOI:10.1161/CIRCOUTCOMES.111.000015. Available at: http://circoutcomes.ahajournals.org.
2. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: a population-based study. BMJ 2013; DOI: 10.1136/bmj.f2610. Available at: http://www.bmj.com.
-------------------
Risk of incident diabetes among patients treated with statins: population based study
BMJ (Published 23 May 2013)
What is already known on this topic
· Given the widespread use of statins to manage hypercholesterolemia, small effects in their efficacy and safety profiles can have important population impact.
· Statins have previously been associated with an increased risk of incident diabetes, though there is controversy around whether this risk differs among drugs
What this study adds
· When compared with pravastatin, atorvastatin, rosuvastatin, and simvastatin are associated with a greater risk of new onset diabetes, regardless of their use for primary or secondary prevention of cardiovascular events
· The risk for rosuvastatin users might depend on the dose prescribed
Abstract
Objective To examine the risk of new onset diabetes among patients treated with different HMG-CoA reductase inhibitors (statins).
Design Population based cohort study with time to event analyses to estimate the relation between use of particular statins and incident diabetes. Hazard ratios were calculated to determine the effect of dose and type of statin on the risk of incident diabetes.
Setting Ontario, Canada.
Participants All patients aged 66 or older without diabetes who started treatment with statins from 1 August 1997 to 31 March 2010. The analysis was restricted to new users who had not been prescribed a statin in at least the preceding year. Patients with established diabetes before the start of treatment were excluded.
Interventions Treatment with statins.
Main outcome measure Incident diabetes.
Results Compared with pravastatin (the reference drug in all analyses), there was an increased risk of incident diabetes with atorvastatin (adjusted hazard ratio 1.22, 95% confidence interval 1.15 to 1.29), rosuvastatin (1.18, 1.10 to 1.26), and simvastatin (1.10, 1.04 to 1.17). There was no significantly increased risk among people who received fluvastatin (0.95, 0.81 to 1.11) or lovastatin (0.99, 0.86 to 1.14). The absolute risk for incident diabetes was about 31 and 34 events per 1000 person years for atorvastatin and rosuvastatin, respectively. There was a slightly lower absolute risk with simvastatin (26 outcomes per 1000 person years) compared with pravastatin (23 outcomes per 1000 person years). Our findings were consistent regardless of whether statins were used for primary or secondary prevention of cardiovascular disease. Although similar results were observed when statins were grouped by potency, the risk of incident diabetes associated with use of rosuvastatin became non-significant (adjusted hazard ratio 1.01, 0.94 to 1.09) when dose was taken into account.
Conclusions Compared with pravastatin, treatment with higher potency statins, especially atorvastatin and simvastatin, might be associated with an increased risk of new onset diabetes.
Introduction
Hydroxymethyl glutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are among the most widely prescribed drugs, with established benefits in patients at risk of cardiovascular events.1 Although statins are tolerated well by most patients, an association with new onset diabetes has recently been suggested.2 In the JUPITER (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin) trial, rosuvastatin was associated with a 27% increased risk of new onset diabetes compared with placebo.3 An increased risk compared with placebo was also observed with atorvastatin and simvastatin.4 5 6 7 In contrast, the West of Scotland coronary prevention study (WOSCOPS) suggested that patients taking pravastatin faced a 30% lower risk of diabetes compared with placebo (relative risk 0.7, 95% confidence interval 0.5 to 0.99).8
In light of these discordant results, several meta-analyses have attempted to characterize the risk of new onset diabetes during treatment with statins.9 10 11 12 13 Limited data, however, exist for direct comparisons of individual statins.11 12 14 Despite these conflicting findings, some evidence supports the notion that different statins might impart differential risks of diabetes.2 7 15 In animal models, pravastatin has been shown to increase adiponectin, improving insulin sensitivity and inhibiting gluconeogenesis, while simvastatin reduces insulin secretion, and atorvastatin and lovastatin impair glucose tolerance.2 7 15 16 For these reasons, in February 2012 the United States Food and Drug Administration mandated labeling changes for all statins except pravastatin.17 18
We examined the healthcare records of more than 1.5 million older people from Ontario, Canada, to examine the association between individual statin use and new onset diabetes.
Discussion
In this population based study, we found that patients treated with atorvastatin, rosuvastatin, or simvastatin were at increased risk of new onset diabetes compared with those treated with pravastatin. No such risk was evident with fluvastatin or lovastatin. The risk associated with rosuvastatin could depend on dose and duration of treatment. The risk of incident diabetes was similar whether statins were being used for primary or secondary prevention.
Overall, we observed a 10-22% increased risk of diabetes for some statins that is consistent with findings from previously published meta-analyses of clinical trials. The increased risk with rosuvastatin significantly decreased after covariate adjustment and became non-significant once dose was taken into consideration. This could possibly represent greater use of rosuvastatin in patients with lower cardiovascular risk.3 In 2009, an analysis of five placebo controlled trials (n=57 593) found a 13% (relative risk 1.13, 95% confidence interval 1.03 to 1.23) increased incidence of diabetes in people taking statins compared with placebo over an average 3.9 years of follow-up,9 with a subsequent analysis of 13 placebo controlled trials (n=91 140) showing a 9% (odds ratio 1.09, 95% confidence interval 1.02 to 1.17) increased incidence of diabetes over four years of follow-up.10 More recently, two meta-analyses have suggested a dose dependent effect for patients receiving high dose atorvastatin or simvastatin treatment versus moderate dose treatment (odds ratio 1.12, 95% confidence interval 1.04 to 1.22) and when considering only atorvastatin trials.11 12 Our results differ from those of the Women's Health Initiative study, which showed a nearly 50% increase in new onset diabetes with statins compared with placebo (hazard ratio 1.48, 95% confidence interval 1.38 to 1.59), with no differential risk among low potency (fluvastatin, lovastatin, pravastatin) and high potency (simvastatin, atorvastatin) statins.14 Our findings, however, are consistent with the findings of Zaharan and colleagues in 2012, who found an increased risk with atorvastatin (hazard ratio 1.25, 1.21 to 1.28), rosuvastatin (1.42, 1.33 to 1.52), and simvastatin (1.14, 1.06 to 1.23).30 Our population based assessment adds to the discussion of risk when doctors are considering starting statin treatment in a patient for primary versus secondary prevention.
Possible mechanisms
Several factors could explain the increased risk of new onset diabetes among patients receiving certain statins.2 7 15 The increased production of plasma derived low density lipoprotein (LDL) cholesterol as a compensatory response to de novo cholesterol synthesis inhibition might result in direct inflammation and oxidation within the ß cell. Consequently, the functional and structural integrity of ß cells is compromized, impairing insulin secretion as a result of cellular apoptosis.15 Additionally, metabolic receptor effects interfere with cellular glucose uptake, energy production, and insulin secretion.2 7 15 16 Statins can also inhibit calcium mediated pancreatic insulin release and decrease expression of the ß cell glucose transporters GLUT-2 and GLUT-4.15 Finally, statins are also known to interfere with the synthesis of ubiquinone (CoQ10), which could independently alter insulin secretion.15 16 The degree to which statins are involved in these respective mechanisms of diabetes onset is variable and supports why some statins pose a higher risk than pravastatin.7 As shown in our dose and potency analyses, the risk could be greater for atorvastatin and simvastatin, regardless of the dose prescribed.
Limitations and strengths
Some limitations of our study merit emphasis. We could not identify and account for potentially important risk factors for diabetes such as weight, ethnicity, and family history. Newer statins might be preferentially used in patients at higher risk of diabetes, though the characteristics of patients in our study were highly similar across study groups. Secondly, data on blood lipids, hemoglobin A1C concentration, or triglyceride concentrations were unavailable, and therefore we could not use these measures for risk stratification or diagnostic purposes. The ODD, however, has been shown to be both sensitive (86-90%) and specific (92-97%).19 Furthermore, we had no data on marketing or promotional efforts nor did we have data on physicians' preferences for particular statins. Although the statin groups were well balanced with respect to a wide variety of demographic and clinical variables, we cannot exclude the possibility of residual confounding.
Our study also had several strengths including a large sample size, use of pravastatin as an active comparator reference group, and a population based design. Our findings suggest that older patients treated with certain statins are at increased risk for incident diabetes, regardless of dose or whether treatment is used for primary or secondary prevention. The risk seems to be greatest with atorvastatin, rosuvastatin, and simvastatin. After adjustment for dose, however, the risk did not seem to persist among rosuvastatin users. Clinicians should consider this risk when they are contemplating statin treatment for individual patients. Preferential use of pravastatin, and potentially fluvastatin or lovastatin, while recognizing the limited efficacy data and increased risk of drug interactions with these two agents, might be warranted. Pravastatin might have a preferential benefit among primary prevention patients at high risk of diabetes.
Results
Patients' characteristics
Over the 14 year study period, we identified 471 250 patients with no history of diabetes who were newly treated with a statin. Of these, 227 994 (48.3%) received a statin for primary prevention, while 243 256 (51.7%) received a statin for secondary prevention. The median age at the outset of treatment was 73 (interquartile range 69-78) and 254 915 (54.1%) were women. Atorvastatin accounted for more than half of all new statin prescriptions, followed by rosuvastatin, simvastatin, pravastatin, lovastatin, and fluvastatin. Tables 1 and 2 show the characteristics of people in the study⇓ ⇓. No major imbalances were apparent among the different statin cohorts.
Primary analysis
The crude event rate for incident diabetes was highest for atorvastatin (30.70 outcomes per 1000 person years) and rosuvastatin (34.21 outcomes per 1000 person years) compared with pravastatin (22.64 outcomes per 1000 person years). Simvastatin (26.22 outcomes per 1000 person years), fluvastatin (21.52 outcomes per 1000 person years), and lovastatin (21.80 outcomes per 1000 person years) had crude event rates similar to pravastatin (table 3⇓). After adjustment for known confounders, and compared with patients treated with pravastatin, those treated with atorvastatin faced a 22% increase in the risk of new onset diabetes (adjusted hazard ratio 1.22, 95% confidence interval 1.15 to 1.29). We also observed a significantly increased risk among those treated with rosuvastatin (1.18, 1.10 to 1.26) and simvastatin (1.10, 1.04 to 1.17) compared with pravastatin (table 3⇓). In contrast, treatment with fluvastatin (0.95, 0.81 to 1.11) or lovastatin (0.99, 0.86 to 1.14) was not significantly associated with an increased risk of incident diabetes.
The figure shows the cumulative incidence of diabetes according to individual statin use⇓. We observed consistent findings when the definition of diabetes included the initiation of antidiabetic drugs, a prescription for blood glucose test strips, or diagnosis in the Ontario Diabetes Database (table 4⇓).
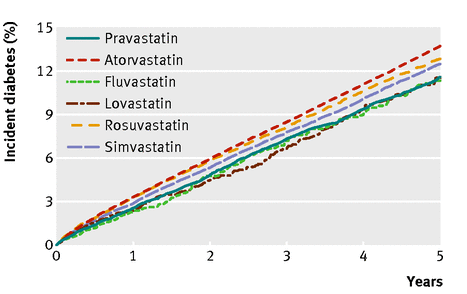
Adjusted survival curves for incident diabetes among new users of statins
Subgroup analyses
We found consistent results in subgroup analyses examining the use of statins for primary and secondary prevention. Relative to pravastatin, treatment with atorvastatin (adjusted hazard ratio 1.20, 95% confidence interval 1.10 to 1.30), rosuvastatin (1.12, 1.02 to 1.23), or simvastatin (1.12, 1.02 to 1.23) was associated with a significantly increased risk of new onset diabetes in the primary prevention cohort, while no increased risk was observed for patients treated with lovastatin (0.98, 0.79 to 1.22) or fluvastatin (1.01, 0.82 to 1.23). Similar findings were observed among the secondary prevention users (table 3⇑).
Overall, moderate (adjusted hazard ratio 1.22, 95% confidence interval 1.19 to 1.26) and high doses (1.30, 1.20 to 1.40) were associated with an increased risk of incident diabetes compared with low doses of statins (table 5⇓). At the individual statin level, our findings were largely consistent with our primary analyses after adjustment for dose. While we found similar risk estimates for simvastatin (1.11, 1.04 to1.17), we observed a slightly attenuated risk for atorvastatin (1.12, 1.05 to 1.18). The risk of incident diabetes among those treated with rosuvastatin, however, was no longer significant after adjustment for dose (1.01, 0.94 to 1.09). When we considered potency, our findings were consistent with our primary analyses (high potency statins (rosuvastatin, atorvastatin) adjusted hazard ratio 1.22, 1.15 to 1.29; moderate potency statins (simvastatin) 1.11, 1.04 to 1.18; low potency statins (fluvastatin, lovastatin) 0.97, 0.87 to 1.09; table 6⇓).
| |
| |
| |
|
|
|