| |
Incidence of AIDS-defining cancers and virus-related and non-virus-related non-AIDS-defining cancers among HIV-infected patients compared with the general population in a large health district of northern Italy, 1999-2009
|
| |
| |
Download the PDF here
HIV Medicine September 2013
A Calabresi,1 A Ferraresi,1 A Festa,2 C Scarcella,3 F Donato,2 F Vassallo,3 RM Limina,2 F Castelli1 and E Quiros-Roldan1 for the Brescia HIV Cancer Study Group*
1University Division of Infectious and Tropical Diseases, University of Brescia, Brescia Spedali Civili General Hospital, Brescia, Italy, 2Institute of Hygiene, Epidemiology and Public Health, University of Brescia, Brescia, Italy and 3Local
Health Authority of the Brescia Province, Brescia, Italy
"An increased risk (SIR = 4.2) of cancers overall was found in HIV-infected patients. .....The risk of all cancers was 4-fold higher in HIV-infected patients than in the reference population. An excess of all malignancies in HIV-infected individuals in the cART era was also observed in Switzerland [28] and in the USA [6], but reported SIRs were lower than in our cohort [SIR = 3.0 (95% CI 2.6-3.6) and SIR = 1.9 (95% CI 1.8-2.1), respectively]. The Bonn cohort in Vogel et al. seems to be more similar to ours [25], with the risk in HIV-infected patients in the Vogel et al. study being four times higher in men [SIR = 4.4 (95% CI 3.6-5.3)] and about three times higher in women [SIR = 2.5 (95% CI 1.4-3.9)], relative to the general population.......Patterns of diseases among HIV-infected patients during the cART era are changing, with increased frequencies of non-AIDS-related conditions, such as tumours, end-stage liver diseases, cardiovascular diseases, severe infections and kidney diseases [29-31]. For malignancies, patterns of cancer incidence are also changing. Our results confirm previous reports that the incidence of ADCs has been reduced in the cART era [32], whereas a steady increase in the incidence of NADCs has been observed over the same period [33]. Actually, NADCs account for more than 50% of all cancers developing in HIV-infected persons [6] (51.9% in our cohort). Although other HIV-infected cohort studies have reported similar observations and suggest that the incidence of NADCs is on the rise in the potent cART era, the incidence of certain non-virus-related NADCs does not seem to show the same trend. In our cohort study, as in the few other studies that have used this classification......
......During the observation period, 416 tumours were observed in 390 patients; 22 patients had a second malignancy and two patients had three different cancers.
Two hundred of the cancers (48.1%) were ADCs, while 138 (33.2%) were non-virus-related NADCs and 78 (18.7%) were virus-related NADCs. The most frequent ADC was KS, accounting for 96 cases, followed by NHL (n = 95) and ICC (n = 9). Among non-virus-related NADCs, the most frequent were skin non-melanoma (n = 41) and trachea/lung cancers (n = 23), while among the virus-related NADCs, HCC accounted for 34 and HL for 31 cases.......
......At the time of first cancer diagnosis, patients with non-virus-related NADCs were older than those with ADCs and with virus-related NADCs (P < 0.001) and they had higher CD4 cell counts (P < 0.001) and higher CD4 nadirs (P < 0.05) (Table 1). Patients with ADCs had the highest HIV viral loads (P < 0.05), and cART was less frequently received in this group (P < 0.001) (Table 1)......
......Older age and severe immunodeficiency were associated with an increased risk for all classes of cancer in our study. Ageing of HIV-infected patients as a result of the increased life expectancy provided by effective cART has increased the risk of many diseases, including malignancies. The accelerated ageing process that occurs in the immune system, also known as "immunosenescence", during HIV infection could account for the higher risk of cancer in people living with HIV infection. The association of virus-related NADCs with low CD4 cell counts suggests that a defect in immunosurveillance on infection with oncogenic agents is permissive for malignant transformation."
Objectives
The aim of the study was to investigate the incidence of AIDS-defining cancers (ADCs) and virus-related and non-virus-related non-AIDS-defining cancers (NADCs) in HIV-infected patients compared with the general population, and to assess the risk factors associated with these malignancies.
Methods
We performed a retrospective cohort study for the period from 1999 to 2009 of HIV-infected patients residing in the Local Health Authority of Brescia (northern Italy). Observed cancers in patients with HIV infection were compared with expected cancers in the population living in the same area using standardized incidence ratios (SIRs). Risk factors were assessed using Poisson regression analysis.
Results
A total of 5090 HIV-infected patients were included in the study, with 32 390 person-years of follow-up. We recorded 416 tumours in 390 HIV-infected patients. Two hundred of these (48.1%) were ADCs, 138 (33.2%) were non-virus-related NADCs and 78 (18.7%) were virus-related NADCs. An increased risk (SIR = 4.2) of cancers overall was found in HIV-infected patients. A large excess of ADCs (SIR = 31.0) and virus-related NADCs (SIR = 12.3) was observed in HIV-infected patients, while the excess risk for non-virus-related NADCs was small (SIR = 1.6). The highest SIRs were observed for Kaposi sarcoma among ADCs and for Hodgkin lymphoma among virus-related NADCs. Conversely, among non-virus-related NADCs, SIRs for a broad range of malignancies were close to unity. In multivariate analysis, increasing age and CD4 cell count < 50 cells/μL were the only factors independently associated with all cancers.
Conclusions
Among HIV-infected people there was an excess of ADCs and also of NADCs, particularly those related to viral infections. Ageing and severe immunodeficiency were the strongest predictors.
Introduction
The introduction of potent combination antiretroviral therapy (cART) has markedly reduced the incidence of opportunistic infections and, among AIDS-defining cancers (ADCs), of Kaposi sarcoma (KS) and non-Hodgkin lymphoma (NHL) [1, 2]. Conversely, cART has had little impact on the incidence of non-AIDS-defining cancers [1-4] (NADCs): Hodgkin lymphoma (HL), invasive anal carcinoma, lung cancer, hepatocellular carcinoma (HCC) and oropharyngeal malignancies have all been diagnosed at higher rates in HIV-infected patients than in the general population [5, 6], as well as melanoma, leukaemia, and vaginal, colon, rectal and kidney cancers according to some studies [5].
Both increased life expectancy [7] and the reduction of competing causes of death have contributed to the increased incidence of NADCs [8, 9], but the longer survival time as a result of cART only partially explains the increasing incidence of these malignancies. The pathogenesis of these tumours is highly variable: traditional cancer risk factors such as smoking [1, 10, 11] and alcohol consumption [10, 12] may contribute to the development of cancer. At the same time, HIV itself may play a role through a direct oncogenic effect (e.g. via the tat gene) [13] or as a consequence of immunosuppression.
In addition, several oncogenic viruses are thought to cause ADCs and NADCs: Epstein-Barr virus (HL, NHL, nasopharyngeal carcinoma and Burkitt's lymphoma) [14], human herpes virus 8 (KS and primary effusion lymphoma) [14], hepatitis C and hepatitis B viruses (HCC) [15] and human papilloma virus (HPV) (carcinoma of the cervix, vulva, vagina, penis, anus and oral cavity, oropharyngeal cancer and in particular tonsillar cancer) [16] have been recognized by the World Health International Agency for Research on Cancer (IARC) as potentially oncogenic viruses.
However, the incidence of cancers among HIV-infected persons and the risk factors associated with them have been less well studied, and some findings are contradictory. Moreover, many of the previous studies grouped all NADCs together and did not divide them according to their viral aetiology.
The main aims of this population-based research were to investigate the incidence of cancers in a single-centre cohort of HIV-infected patients and to investigate the risk factors associated with the development of ADCs, virus-related NADCs and non-virus-related NADCs in the cART era, as compared with the general population living in the same area.
Methods
We conducted a retrospective cohort study for the period from January 1999 to December 2009 of HIV-infected patients residing in the Local Health Authority (LHA) of Brescia (northern Italy) and receiving care for HIV infection at the University Division of Infectious and Tropical Diseases of the University of Brescia or at the Infectious Diseases Department, Brescia Spedali Civili General Hospital. We started our study from January 1999 because data on cancer incidence in the Cancer Registry of Brescia were available from that year, when considering the cART era.
At their first contact with our centre, all patients provided written informed authorization to collect information for scientific purposes and research.
Sociodemographic characteristics, laboratory test results, treatments for HIV/AIDS, and complications including opportunistic infections, cancer and death were recorded on a regular basis and stored in the electronic database utilized for routine clinical management. Cancer diagnoses were retrieved from this electronic database and completed with those recorded in the administrative database of the LHA of Brescia and that of the population-based Cancer Registry of the LHA: the details of this record linkage have been described elsewhere [17]. All cases of cancer were defined on the basis of histological or cytological examination.
Multiple primaries, i.e. cancers of different types occurring in the same subject, were included in the analysis and each cancer was analysed as a single case. We excluded malignancies already diagnosed before the start of observation or before the diagnosis of HIV infection.
Cancer type and site were coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th revision (World Health Organization, 1992).
We categorized malignancies as ADCs [NHL, KS and invasive cervical carcinoma (ICC)] and NADCs. We further divided NADCs into two a priori categories: virus-related (HCC, HL, cancers of the vagina, vulva and penis, squamous cell anal cancer and certain squamous cell oral cavity/pharynx cancers, as defined by Chaturvedi et al. [18]) and non-virus-related (all the others).
For each person included in the study, the relevant time period for the calculation of person-years at risk began on 1 January 1999 or at the date of enrolment in the cohort, if later, and ended at cancer diagnosis, at the last follow-up visit, on 31 December 2009 or at death.
Expected numbers of cancers were computed on the basis of the incidence rates of the Cancer Registry of Brescia LHA for the periods 1999-2001 and 2004-2006, standardized by age. Observed cancers included incident cases in HIV-infected patients recorded during the study period. Observed numbers of cancers were compared with expected numbers by calculating standardized incidence ratios (SIRs), and their corresponding 95% confidence intervals (CIs), using the Poisson distribution.
Incidence rates (IRs) were calculated by dividing the number of observed cases by the corresponding person-years at risk and were standardized for age, using the direct method [19] with the European population as the standard.
Poisson regression analysis was used to evaluate the univariate and multivariate associations between covariates and cancers. A P-value of 0.05 was used for all the two-tailed statistical tests, although P-values close to the threshold are also shown. Age, race and sex were included in the fitted models as possible confounders, regardless of statistical significance. For all other variables, only those with a P-value < 0.05 in univariate analysis were included in the multivariate model. A P-trend linear analysis was also performed when considering CD4 cell count.
All analyses were performed using stata 12 (StataCorp LP, College Station, TX).
This study involving human subjects was performed in accordance with the Helsinky Declaration of 1975 as revised in 2000 and it was approved by the local ethical committee of the Spedali Civili General Hospital of Brescia.
Results
A total of 5090 HIV-infected patients were included in the study, with 32 390 person-years of follow-up (median 6.44 person-years).
Table 1 reports the baseline characteristics of the patients. They were mainly Italian (81.9%) and male (71.9%), with a median age of 35 years (range 19-64 years). A large proportion (42.9%) acquired HIV through injecting drug use and 45.8% were coinfected with a hepatitis virus. Median CD4 cell count was 365 cells/μL (range 11-1190 cells/μL) and one-third of patients (33.6%) were on cART. Only 887 patients (17.43%) had an AIDS diagnosis (not including AIDS-defining malignancies) before baseline.
During the observation period, 416 tumours were observed in 390 patients; 22 patients had a second malignancy and two patients had three different cancers. Two hundred of the cancers (48.1%) were ADCs, while 138 (33.2%) were non-virus-related NADCs and 78 (18.7%) were virus-related NADCs. The most frequent ADC was KS, accounting for 96 cases, followed by NHL (n = 95) and ICC (n = 9). Among non-virus-related NADCs, the most frequent were skin non-melanoma (n = 41) and trachea/lung cancers (n = 23), while among the virus-related NADCs, HCC accounted for 34 and HL for 31 cases.
At the time of first cancer diagnosis, patients with non-virus-related NADCs were older than those with ADCs and with virus-related NADCs (P < 0.001) and they had higher CD4 cell counts (P < 0.001) and higher CD4 nadirs (P < 0.05) (Table 1). Patients with ADCs had the highest HIV viral loads (P < 0.05), and cART was less frequently received in this group (P < 0.001) (Table 1).
The mean incidence rate was 61.7/10 000 person-years for ADCs, which was higher than the mean incidence rates for non-virus-related NADCs (42.6/10 000 person-years) and virus-related NADCs (24.1/10 000 person-years).
Observed and expected numbers of cancers and corresponding SIRs are shown for cancer types or sites with at least two cases observed (Table 2). An increased risk (SIR = 4.2) in the HIV-infected population was found when all cancers were considered, and the risk in men was almost double that in women. An excess of ADCs (SIR = 31.0) and virus-related NADCs (SIR = 12.3) was observed.
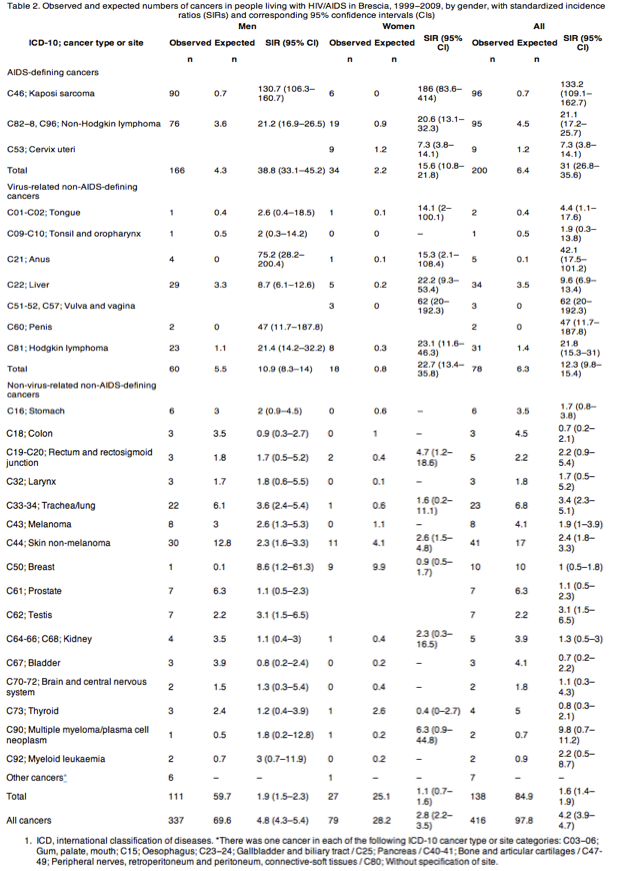
Among ADCs, the highest SIR was observed for KS, followed by NHL and ICC, and among virus-related NADCs, the SIR for HL was more than 20 (Table 2).
Interestingly, among non-virus-related NADCs, SIRs for a broad range of common malignancies, such as cancers of the breast, prostate, colon, thyroid and urinary tract, were close to unity or lower (Table 2).
We also examined cancer incidence trends over time. The age-standardized incidence rate of all cancers increased from 1999 to 2009 (Fig. 1a). Considering cancers divided into the three categories, there was a decrease in the age-standardized incidence rate of ADCs (Fig. 1b), but an increase in the rates of both virus-related and non-virus-related NADCs (Fig. 1c and d, respectively).
The P-trend linear analysis for CD4 cell count category gave significant results in univariate and multivariate analyses for ADCs (P < 0.001) as well as for NADCs, both virus-related (P < 0.05) and non-virus-related (P < 0.05) (data not shown).
Discussion
This was a population-based, registry-linkage study of individuals with HIV infection or AIDS, residing in the LHA of Brescia, who were followed between 1999 and 2009. It was based on a single-centre cohort, but we would like to point out that this LHA has a population of more than 1.1 million inhabitants [20] and has the highest incidence of AIDS among the provinces of northern Italy [21].
Cases of cancer were reported to our registry through passive and active surveillance systems [17]. The record linkage among three different sources allowed us to obtain records of cancer cases missing from our electronic clinic database (about 20% of all diagnoses [17]) and provided detailed clinical information.
Here, all patients infected with HIV, and not only those who developed AIDS, were considered, while other Italian epidemiological studies used a procedure of record linkage between the Italian AIDS Registry and Cancer Registries [22, 23]. Only one Italian cohort study [24] reported the incidence of malignancies in a cohort of HIV-infected patients; in that study, cancer cases were recorded during clinical visits and a comparison with the general population was not performed.
The old division between ADCs and NADCs has been widely used [1-6], and some recent studies divided malignancies into infection-related types and cancers unrelated to infectious diseases [25, 26]. In this study, a categorization in which cancers were divided into ADCs, virus-related NADCs and non-virus-related NADCs was used. Similarly to our study, Reekie et al. [27] divided NADCs into virus-related and non-virus-related types, but they included in the first group also cancers for which a viral aetiology is only suspected (e.g. cancer of the larynx) and they presumptively considered all oral cancers as HPV-related.
The SIRs in this study are particularly accurate and valid, as the incidence of cancers in our cohort was compared with the incidence rates from the Cancer Registry of Brescia, where our centre is located and which was the legal place of residency reported by all patients included in the study.
The risk of all cancers was 4-fold higher in HIV-infected patients than in the reference population. An excess of all malignancies in HIV-infected individuals in the cART era was also observed in Switzerland [28] and in the USA [6], but reported SIRs were lower than in our cohort [SIR = 3.0 (95% CI 2.6-3.6) and SIR = 1.9 (95% CI 1.8-2.1), respectively]. The Bonn cohort in Vogel et al. seems to be more similar to ours [25], with the risk in HIV-infected patients in the Vogel et al. study being four times higher in men [SIR = 4.4 (95% CI 3.6-5.3)] and about three times higher in women [SIR = 2.5 (95% CI 1.4-3.9)], relative to the general population.
Patterns of diseases among HIV-infected patients during the cART era are changing, with increased frequencies of non-AIDS-related conditions, such as tumours, end-stage liver diseases, cardiovascular diseases, severe infections and kidney diseases [29-31]. For malignancies, patterns of cancer incidence are also changing. Our results confirm previous reports that the incidence of ADCs has been reduced in the cART era [32], whereas a steady increase in the incidence of NADCs has been observed over the same period [33]. Actually, NADCs account for more than 50% of all cancers developing in HIV-infected persons [6] (51.9% in our cohort). Although other HIV-infected cohort studies have reported similar observations and suggest that the incidence of NADCs is on the rise in the potent cART era, the incidence of certain non-virus-related NADCs does not seem to show the same trend. In our cohort study, as in the few other studies that have used this classification, SIRs for common malignancies such as breast, prostate, colon, thyroid and urinary tract cancer were close to unity. These findings suggest that a more accurate classification must be used in epidemiological studies of malignancies in HIV-infected individuals.
Older age and severe immunodeficiency were associated with an increased risk for all classes of cancer in our study. Ageing of HIV-infected patients as a result of the increased life expectancy provided by effective cART has increased the risk of many diseases, including malignancies. The accelerated ageing process that occurs in the immune system, also known as "immunosenescence", during HIV infection could account for the higher risk of cancer in people living with HIV infection. The association of virus-related NADCs with low CD4 cell counts suggests that a defect in immunosurveillance on infection with oncogenic agents is permissive for malignant transformation. Several variables have been used to study the relationship between cancer and immunodeficiency, resulting in conflicting findings depending on the variable used (i.e. CD4 count nadir, baseline CD4 count, time-updated CD4 count or cumulative time with a given CD4 count), and there is not a unique, commonly accepted and clear "marker" of immunodeficiency that is associated with the cancer risk.
Finally, cART use for less than 6 months before cancer diagnosis was associated with an increased risk of ADCs and non-virus-related NADCs but not virus-related NADCs. Recently, data have been published suggesting a direct protective effect of cART on ADCs, independent of the cART effect on CD4 cell count [34]. The effect of cART use on the risk of NADCs seems to be more complicated, and conflicting data have been reported in the literature: some studies showed a protective effect of cART on the risk of NADCs [35, 36] and others reported no association [1, 22], while Powles et al. [37] found an adverse association between exposure to nonnucleoside reverse transcriptase inhibitors (NNRTIs) and risk of HL. The conflicting results may be attributable to the differences in the definition of cART use (e.g. in terms of the duration of use, cART type or the dose-response relationship with the duration of use) or differences in the classification or type of NADCs studied. Chao et al. [34] did not find a clear association between ART [any ART use, the cumulative duration of any ART use, or use of protease inhibitors (PIs) or NNRTIs] and the risk of infection-related or infection-unrelated NADCs, lung cancer or HL.
The current study has weaknesses and strengths. Data were lacking on important cancer cofactors such as smoking behaviour, alcohol use, family history of cancer and biomarkers to detect Epstein-Barr virus and HPV. In addition, we had no data on cancer screening practices and we could not therefore explore their influence on cancer incidence over time. Finally, all patients were observed exclusively in the cART era (1999 to 2009). Despite these limitations, our study has several strengths, which include the high-quality case ascertainment of cancer diagnoses, the duration and completeness of follow-up, and the good representation of women (almost 30%) and in different HIV-transmission categories.
Nevertheless, given the variability between cohorts in risk factors for HIV infection, oncogenic infections, and genetic and environmental factors, continuing evaluation of cancer risk in this population is needed.
| |
| |
| |
|
|
|