| |
The CD4:CD8 ratio is associated with markers of age-associated disease in virally suppressed HIV-infected patients with immunological recovery
|
| |
| |
Download the PDF here
".......In multivariate analyses, inversion of the CD4:CD8 ratio was independently associated with higher IMT, arterial stiffness and lower eGFR. Importantly, these associations remained statistically significant when the sample was censored by low nadir CD4 count (< 200 cells/μL) and low CD4 T-cell count (< 500 cells/μL), suggesting that the CD4:CD8 ratio provides additional information to that provided by nadir CD4 and CD4 T-cell counts as surrogate markers of age-associated disease [27, 28]. We also observed that men and older patients more frequently showed inversion of the CD4:CD8 ratio, a finding that is consistent with those of studies in the general population [29, 30]."
-----------------------------
Low CD4/CD8 Ratio Predicts Non-AIDS Morbidity, Mortality in ART Responders
7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur
http://natap.org/2013/IAS/IAS_04.htm........To explore the power of the CD4/CD8 ratio, CD4 count, CD8 count, and nadir CD4 count to predict non-AIDS events, the researchers calculated the area under the curve (AUC) for each variable. The CD4/CD8 ratio had the highest AUC (0.720, 95% CI 0.662 to 0.777), significantly higher than AUCs for CD4 count, CD8 count, or nadir CD4 count (P < 0.001).A CD4/CD8 ratio of 0.4 proved the most accurate cutoff for predicting non-AIDS events.
HIV Medicine (Sept 6 2013)
S Serrano-Villar,1,2 S Moreno,1,2 M Fuentes-Ferrer,3 C Sanchez-Marcos,4 M avila,4 T Sainz,5,6 NGP de Villar,7
A Fernandez-Cruz3 and V Estrada3
1Infectious Diseases Department, University Hospital Ramon y Cajal, Madrid, Spain, 2Health Research Institute Ramon y
Cajal (IRYCIS), Madrid, Spain, 3Preventive Medicine Department, University Hospital Cl’nico San Carlos, Madrid, Spain,
4Internal Medicine Department, University Hospital Cl’nico San Carlos, Madrid, Spain, 5Molecular Immune-Biology
Laboratory, University Gregorio Maranon, Madrid, Spain, 6Health Research Institute Gregorio Maranon (IiSGM), Madrid, Spain and 7Internal Medicine Department, University Hospital of Fuenlabrada, Madrid, Spain
"HIV-infected subjects exhibit various changes in the adaptive immune system that are shared by elderly people. Thus, different terms such as 'inflamm-aging' and immunosenescence have been proposed to allude to this immune phenotype that characterizes both HIV-infected and elderly persons [4, 8]. Outside HIV infection, inversion of the CD4:CD8 ratio (< 1), also termed the immune risk profile, is considered a surrogate marker of immunosenescence and independently predicts all-cause mortality [9-12]. Notably, most ART-naïve patients show a low CD4:CD8 T-cell ratio before starting ART, which progressively increases as CD4 counts rise after ART initiation. However, an appreciable number of individuals on long-term treatment still show a low ratio despite CD4 count normalization. In the light of what is known about the CD4:CD8 ratio in the general population, a possible explanation for this finding is the presence of increased immune activation and immunosenescence in these subjects; however, this explanation remains speculative [4]. We have recently reported that the CD4:CD8 ratio is an independent predictor of immune activation in virally suppressed HIV-infected individuals, correlating also with immunosenescence [13]. In a similar analysis in vertically HIV-infected children and adolescents on ART, we found that a low CD4:CD8 ratio was associated with increased levels of CD8 T cells expressing an activation/exhaustion phenotype, and a low naïve : memory T-cell ratio, a feature that also characterizes immunosenescence [14]."
".....inversion of the CD4:CD8 ratio may identify HIV-infected patients with ongoing immunosenescence and, as a consequence, at higher risk of age-associated diseases ........we hypothesized that an inverted CD4:CD8 ratio despite successful ART may be a marker of non-AIDS-related complications. In this context, we aimed to evaluate whether the CD4:CD8 ratio, which can be obtained in most clinical settings and is routinely measured in HIV-infected patients, was associated in treated HIV-infected patients with markers of subclinical atherosclerosis, arterial stiffness, incipient renal impairment or muscle wasting......In this study in virally suppressed, HIV-infected subjects, we explored the associations of the CD4:CD8 ratio with markers of vascular disease, kidney disease and muscle wasting. We found that the CD4:CD8 ratio was negatively correlated with IMT, and positively correlated with eGFR (both values, P < 0.05). In addition, subjects with inversion of the CD4:CD8 ratio more frequently showed higher IMT, lower eGFR and lower muscle mass (all values, P < 0.05). No significant associations were detected with arterial stiffness. In multivariate analyses, inversion of the CD4:CD8 ratio was independently associated with higher IMT, arterial stiffness and lower eGFR. Importantly, these associations remained statistically significant when the sample was censored by low nadir CD4 count (< 200 cells/μL) and low CD4 T-cell count (< 500 cells/μL), suggesting that the CD4:CD8 ratio provides additional information to that provided by nadir CD4 and CD4 T-cell counts as surrogate markers of age-associated disease [27, 28]. We also observed that men and older patients more frequently showed inversion of the CD4:CD8 ratio, a finding that is consistent with those of studies in the general population [29, 30]."
Abstract
Objectives
Inversion of the CD4:CD8 ratio (< 1) has been identified as a hallmark of inmmunosenescence and an independent predictor of mortality in the general population. We aimed to assess the association between the CD4:CD8 ratio and markers of age-associated disease in treated HIV-infected patients with good immunovirological response.
Methods
A cross-sectional analysis was conducted in 132 HIV-infected adults on antiretroviral therapy (ART), with plasma HIV RNA < 50 HIV-1 RNA copies/mL for at least 1 year, CD4 count > 350 cells/μL and age < 65 years. We analysed the associations between the CD4:CD8 ratio and subclinical atherosclerosis [assessed using carotid intima-media thickness (IMT)], arterial stiffness [assessed using the augmentation index (AIx)], the estimated glomerular filtration rate (eGFR), muscle wasting and sarcopenia [assessed using appendicular lean mass/height2 (ALM) measured by dual-energy X-ray absorptiometry (DEXA)].
Results
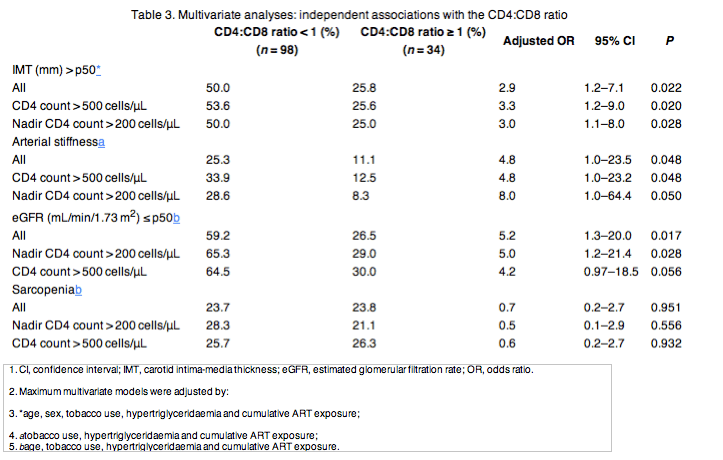
CD4:CD8 ratio inversion was associated with higher IMT, lower eGFR and lower ALM (all values P < 0.05), but not with AIx. In multivariate analyses adjusted for age, sex, hypertriglyceridaemia, tobacco use and cumulative ART exposure, inversion of the CD4:CD8 ratio was independently associated with higher IMT [odds ratio (OR) 2.9; 95% confidence interval (CI) 1.2-7.1], arterial stiffness (OR 4.8; 95% CI 1.0-23.5) and lower eGFR (OR 5.2; 95% CI 1.0-64.4), but not sarcopenia (OR 0.7; 95% CI 0.2-2.7). These associations persisted when models were applied to subjects with nadir CD4 counts > 200 cells/μL and those with CD4 counts > 500 cells/μL.
Conclusions
The CD4:CD8 ratio in treated HIV-infected subjects with good immunovirological response is independently associated with markers of age-associated disease. Hence, it might be a clinically useful predictor of non-AIDS-defining conditions.
Introduction
Antiretroviral therapy (ART) is among the greatest successes of modern medicine, having rapidly changed the prognosis of HIV-infected individuals from years to decades of survival. However, ART has failed in its attempt to completely restore normal health to HIV-infected subjects. Although the reasons remain poorly understood, subjects on successful ART still present increased morbidity and mortality relative to uninfected individuals [1-3]. This shortening of the expected life span has recently been associated with increased risk of so-called 'non-AIDS-related' complications, which include cardiovascular disease, renal impairment, liver disease, neurocognitive disorders, non-AIDS-defining cancers, osteoporosis, muscle wasting and frailty. All these complications are generally associated with aging, and concern has been increasing regarding the possibility that persons living with HIV suffer from an 'accelerated aging' syndrome [4]. Most of these noninfectious conditions have been related to the ongoing immune activation and low-level systemic inflammation that occur in chronic HIV infection despite effective ART [4-7]. Although several studies have described immunovirological factors that predict non-AIDS-related end-organ disease risk, such as immune activation, most of these biomarkers are not available in routine clinical practie.
Interestingly, HIV-infected subjects exhibit various changes in the adaptive immune system that are shared by elderly people. Thus, different terms such as 'inflamm-aging' and immunosenescence have been proposed to allude to this immune phenotype that characterizes both HIV-infected and elderly persons [4, 8].
Outside HIV infection, inversion of the CD4:CD8 ratio (< 1), also termed the immune risk profile, is considered a surrogate marker of immunosenescence and independently predicts all-cause mortality [9-12]. Notably, most ART-naïve patients show a low CD4:CD8 T-cell ratio before starting ART, which progressively increases as CD4 counts rise after ART initiation. However, an appreciable number of individuals on long-term treatment still show a low ratio despite CD4 count normalization. In the light of what is known about the CD4:CD8 ratio in the general population, a possible explanation for this finding is the presence of increased immune activation and immunosenescence in these subjects; however, this explanation remains speculative [4]. We have recently reported that the CD4:CD8 ratio is an independent predictor of immune activation in virally suppressed HIV-infected individuals, correlating also with immunosenescence [13]. In a similar analysis in vertically HIV-infected children and adolescents on ART, we found that a low CD4:CD8 ratio was associated with increased levels of CD8 T cells expressing an activation/exhaustion phenotype, and a low naïve : memory T-cell ratio, a feature that also characterizes immunosenescence [14]. Thus, inversion of the CD4:CD8 ratio may identify HIV-infected patients with ongoing immunosenescence and, as a consequence, at higher risk of age-associated diseases [12]. Hence, we hypothesized that an inverted CD4:CD8 ratio despite successful ART may be a marker of non-AIDS-related complications. In this context, we aimed to evaluate whether the CD4:CD8 ratio, which can be obtained in most clinical settings and is routinely measured in HIV-infected patients, was associated in treated HIV-infected patients with markers of subclinical atherosclerosis, arterial stiffness, incipient renal impairment or muscle wasting.
Methods
Study design, participants, setting and eligibility
We conducted an observational, cross-sectional study of 132 consecutive HIV-infected patients who attended a university-based HIV clinic in Madrid between February and October 2011. Subjects were recruited if they were HIV infected and on stable triple ART, defined as continuous treatment with three antiretroviral drugs including either a nonnucleoside reverse transcriptase inhibitor or a protease inhibitor, had had undetectable plasma HIV RNA levels for at least 1 year, and showed CD4 counts ≥ 350 cells/uL at inclusion in the study. As we aimed to analyse variables associated with subclinical aging, exclusion criteria included age > 65 years and the presence of cardiovascular disease (previous stroke, myocardial infarction or intermittent claudication) or chronic kidney disease. The study conformed to the principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines and was approved by the local Ethics Committee. All patients gave their written informed consent to participate in the study.
Clinical and laboratory measurements
Medical records were carefully reviewed and all subjects underwent a physical examination. Information on gender, age, body mass index, smoking status, family history of cardiovascular disease and treatment with antiretroviral drugs was recorded. The presence of arterial hypertension, hypercholesterolaemia and hypertriglyceridaemia was defined according to the Adult Treatment Panel III criteria [15]. All patients underwent dual-energy X-ray absorptiometry (DEXA) total body composition measurements, and appendicular lean mass (kg)/height2 (m2) (ALM) was used to assess the extent of muscle wasting. We defined sarcopenia as ALM < 2 standard deviations below the mean for young healthy adults (cut-offs were < 7.26 kg/m2 for men and < 5.45 kg/m2 for women) [16, 17]. A sample of fasting venous blood was obtained to determine concentrations of glucose, high-sensitivity C-reactive protein (CRP), interleukin-6, creatinine, total cholesterol, high-density lipoprotein (HDL) cholesterol and triglycerides using standard enzymatic methods. Low-densitylipoprotein (LDL) cholesterol concentrations were calculated using the Friedewald equation [18].
Plasma viral load was measured using the Cobas TaqMan HIV-1 assay (Roche Diagnostics Systems, Branchburg, NJ). CD4 and CD8 T-cell counts were determined by flow cytometry (Beckman-Coulter, Munster, Germany). Plasma levels of CRP were measured using nephelometry (Siemens Healthcare Diagnostics, Deerfield, IL) and interleukin-6 using chemoluminiscence (Siemens Healthcare Diagnostics). The minimum detection limits of the enzyme-linked immunosorbent assays (ELISAs) for CRP and interleukin-6 were 0.011 mg/L and 1.9 pg/mL, respectively. Typical coefficients of variation for these determinations were < 5%.
Renal function assessment
We used the recently developed Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula to calculate the estimated glomerular filtration rate (eGFR), which has proved to be more accurate than the routinely used Modification of Diet in Renal Disease (MDRD) formula, especially in patients with normal kidney function [19].
Measurement of subclinical atherosclerosis
The degree of subclinical atherosclerosis was evaluated by means of the carotid intima-media thickness (IMT), which has been demonstrated to be an important predictor of cardiovascular events in both the general population [20] and HIV-infected individuals [21], following standard procedures previously described [22]. Briefly, IMT was measured using high-resolution ultrasound (HD7 model; Philips Medical Systems, Eindhoven, The Netherlands) at the common carotid artery (1 cm proximal to the bifurcation) and interpreted using the Mannheim criteria [23]. Measurements were performed by two trained technicians who had previously participated in a pilot study (repeated and blinded measurements performed in 29 patients). The intraclass correlation coefficient was > 0.90.
Measurement of arterial stiffness
The augmentation index (AIx) was used as a surrogate marker of arterial stiffness, which correlates with cardiovascular risk [24]. AIx was measued by pulse wave tonometry, following the recommendations of the European Society of Cardiology [25]. Subjects were examined after resting for at least 5 min in a supine position at a room temperature of 22 ± 1°C. No cigarette smoking or caffeine ingestion was allowed 2 h prior to the examination. Arterial stiffness was assessed noninvasively using the SphygmoCor System (AtCor Medical, Sydney, Australia) and peripheral pressure waveforms were recorded from the right radial artery using applanation tonometry for pulse wave analysis. After 10 sequential waveforms had been acquired, a validated generalized transfer function was used to generate the corresponding central aortic pressure waveform, from which AIx was obtained, which was calculated as the ratio between augmentation pressure and pulse pressure. Because AIx is influenced by heart rate, an index normalized for a heart rate of 75 bpm (AIx@75) was calculated using a general transformation function previously described [26]. Higher values of AIx@75 indicate early return of the reflected wave or increased wave reflection from the periphery as a result of increased arterial stiffness. Subjects were diagnosed with arterial stiffness if AIx@75 was below 2 standard deviations from the reference values in a sex- and age-matched reference population.
Statistical analysis
Qualitative variables were summarized as a frequency distribution and normally distributed quantitative variables as mean ± standard deviation. Continuous nonnormally distributed variables were summarized as median and interquartile range (IQR). Means for variables with a normal distribution were compared using the t-test. Nonparametric variables were assessed using the Mann-Whitney test.
Given the nonnormal distribution of some of the variables, the Spearman correlation coefficient was used to analyse the correlation between continuous variables.
Independent associations between the CD4:CD8 ratio [as a binary variable: an inverted CD4:CD8 ratio (< 1) vs. a normal CD4:CD8 ratio (≥ 1)] and binary outcomes of subclinical aging (IMT > or ≤ p50, presence or absence of arterial stiffness as described above, eGFR > or ≤ p50, and presence or absence of sarcopenia as described above) were evaluated in a multivariate analysis. The modelling strategy involved explanatory logistic regression analysis. Variables with an imbalance between the study groups were introduced into the model, as well as those variables that could exert confounding on the association between the CD4:CD8 ratio and the dependent variables. The variables nadir CD4 and CD8 T-cell counts were excluded from the models because of collinearity. Hence, the maximum model was adjusted by age, sex, hypertriglyceridaemia, tobacco use and cumulative ART exposure. Cumulative tenofovir exposure was also included in the model for renal impairment. As arterial stiffness was defined according to sex and age reference values and sarcopenia according to sex, these demographic variables were not included in the multivariate model for each of these two dependent variables. A backward strategy was used, considering a significance level of 0.05 to eliminate variables. The overall model was reapplied including only the confounding factors that, after their elimination from the model, produced a ≤ 10% change in the estimate of the coefficient of the variable of principal exposure (inverted CD4:CD8 ratio). The magnitude of association was evaluated using the odds ratio (OR) and 95% confidence interval (CI). In order to assess whether the CD4:CD8 ratio may provide additional information to CD4 nadir and CD4 counts, we applied the multivariate models to the subgroups of patients with low nadir CD4 counts (< 200 cells/μL) and high CD4 counts (> 500 cells/ μL).
The null hypothesis was rejected with a type I error < 0.05 (α < 0.05). Statistical analyses were performed using stata version 12.0 (StataCorp LP, College Station, TX), and figures were generated using GraphPad Prism 5.00 (GraphPad Software, San Diego, CA).
Results
Table 1 summarizes the main characteristics of the 132 subjects included in the study. The study sample was representative of a middle-aged (47 ± 7 years) HIV-infected population on long-term triple ART (median exposure 7.5 years) and with good immunovirological status (the median CD4 count was 591 cells/mL and 100% of patients had undetectable viral load). Most patients (75%) displayed a low 10-year cardiovascular risk (< 10%), estimated using the Framingham risk score.
All variables assessing subclinical aging - IMT and arterial stiffness for cardiovascular aging, eGFR for renal impairment and ALM as a marker of muscle wasting and sarcopenia - are summarized in Table 1.
Differences in demographic and immunovirological variables, cardiovascular risk factors and inflammation biomarkers according to the CD4:CD8 ratio
Table 2 summarizes the characteristics of patients according to the presence of an inverted (< 1) or normal (≥ 1) CD4:CD8 ratio. A total of 98 patients (74.2%) exhibited inversion of the CD4:CD8 ratio. These individuals were older and were more frequently male, and showed a lower median CD4 count nadir CD4 count nadir compared with patients with a normal CD4:CD8 ratio (all values P < 0.05). No significant differences were found between the two groups in the frequency of cardiovascular risk factors, with the exception of a higher frequency of hypertriglyceridaemia (P < 0.001) in subjects with a low CD4:CD8 ratio. Levels of inflammatory markers (CRP and interleukin-6) were similar between the two groups.
Associations between subclinical aging and the CD4:CD8 ratio
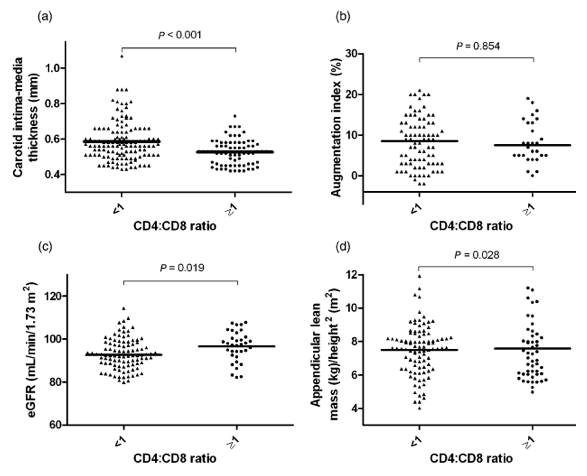
Next, we explored the linear and categorical associations between the CD4:CD8 ratio and variables associated with subclinical aging, reflecting carotid atherosclerosis (IMT), arterial stiffness (AIx), renal impairment (eGFR) and muscle wasting or sarcopenia (ALM). First, we analysed the linear correlations between the CD4:CD8 ratio and IMT, eGFR and ALM (Fig. 1). The CD4:CD8 ratio was inversely correlated with IMT (r = -0.192; P = 0.037) (Fig. 1a), but not with AIx (r = -0.015; P = 0.875) (Fig. 1b), and positively correlated with eGFR (r = 0.215; P = 0.013) (Fig. 1c). No linear correlation with ALM was detected (r = -0.169; P = 0.134) (Fig. 1d). We then performed categorical comparisons according to the presence of a normal or an inverted CD4:CD8 ratio. Subjects with inversion of the CD4:CD8 ratio showed increased IMT (Fig. 2a), lower eGFR (Fig. 2c) and lower ALM (Fig. 2c) (all values P < 0.05). No statistically significant differences were found in AIx values between subjects with normal and inverted CD4:CD8 ratios. Although patients with inversion of the CD4:CD8 ratio exhibited a higher frequency of arterial stiffness, the difference was not statistically significant (11% vs. 25%, respectively; P = 0.122).
Figure 1. Linear correlations between the CD4:CD8 ratio and different markers of age-associated disease. The CD4:CD8 ratio was inversely correlated with carotid intima-media thickness (IMT) (r = -0.192; P = 0.037) (a), but not with the augmentation index (r = -0.015; P = 0.875) (b), and positively correlated with the estimated glomerular filtration rate (eGFR) (r = 0.215; P = 0.013) (c). No linear correlation with appendicular lean mass/height2 (ALM) was detected (r = -0.169; P = 0.134) (d).
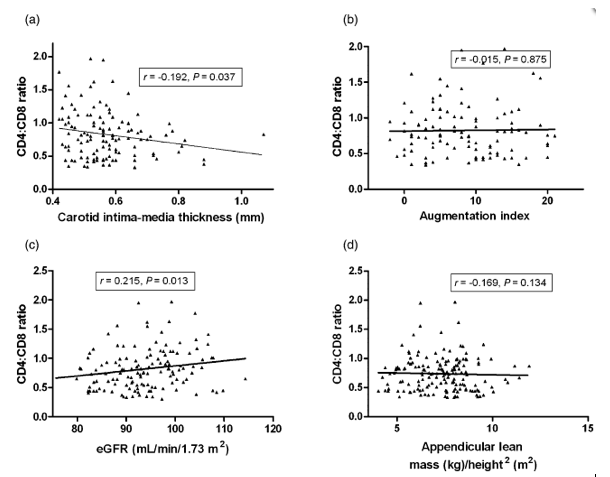
Figure 2. Categorical associations between the CD4:CD8 ratio and markers of age-associated disease. Subjects with inversion of the CD4:CD8 ratio showed increased carotid intima-media thickness (IMT) (a), lower estimated glomerular filtration rate (eGFR) (b) and lower appendicular lean mass/height2 (ALM) (c) (all values P < 0.05). No statistically significant differences were found in augmentation index (AIx) values between subjects with normal and inverted CD4:CD8 ratios. Although patients with inversion of the CD4:CD8 ratio exhibited a higher frequency of arterial stiffness, the difference was not statistically significant (11% vs. 25%, respectively; P = 0.122).
Multivariate analysis
A series of logistic regression models were explored to analyse the association of the CD4:CD8 ratio with variables reflecting subclinical aging (IMT, arterial stiffness, eGFR and sarcopenia), which were used as dependent variables in consecutive multivariate models, adjusting by age, sex, hypertriglyceridaemia, tobacco use and cumulative ART exposure (and specifically tenofovir exposure in the model for renal impairment). In the multivariate analyses, no associations with sarcopenia were observed, but subjects with inversion of the CD4:CD8 ratio showed a significant threefold increased risk of higher IMT and a significant fivefold increased risk of arterial stiffness and lower eGFR (Table 3). Overall, these independent associations and markers of age-associated disease persisted when multivariate models were applied to subjects with nadir CD4 > 200 cells/μL, CD4 count > 500 cells/μL or both.
Discussion
In this study in virally suppressed, HIV-infected subjects, we explored the associations of the CD4:CD8 ratio with markers of vascular disease, kidney disease and muscle wasting. We found that the CD4:CD8 ratio was negatively correlated with IMT, and positively correlated with eGFR (both values, P < 0.05). In addition, subjects with inversion of the CD4:CD8 ratio more frequently showed higher IMT, lower eGFR and lower muscle mass (all values, P < 0.05). No significant associations were detected with arterial stiffness. In multivariate analyses, inversion of the CD4:CD8 ratio was independently associated with higher IMT, arterial stiffness and lower eGFR. Importantly, these associations remained statistically significant when the sample was censored by low nadir CD4 count (< 200 cells/μL) and low CD4 T-cell count (< 500 cells/μL), suggesting that the CD4:CD8 ratio provides additional information to that provided by nadir CD4 and CD4 T-cell counts as surrogate markers of age-associated disease [27, 28]. We also observed that men and older patients more frequently showed inversion of the CD4:CD8 ratio, a finding that is consistent with those of studies in the general population [29, 30].
In recent years, consistent data have led to the widespread assumption that HIV infection leads to an increased risk of 'non-AIDS-related' events. Although the underlying mechanisms are not fully understood, a whole body of research suggests that these adverse outcomes are linked to a remodelling of the immune system. Persistent inflammation and immune activation are widely accepted as the major driving factors of this immune senescence and exhaustion that ultimately lead to disease progression and adverse outcomes [31]. Thus, many groups have focused on the study of biomarkers of inflammation and surrogate markers of non-AIDS-related events during treated HIV infection [32]. However, none of these markers has been properly validated and, despite the fact that viral control and immunological restoration no longer pose a clinical challenge, management of HIV-infected patients is still based on optimization of the same surrogate parameters of immunovirological control as were used 30 years ago: viral load and CD4 T-cell count.
Most studies addressing the problem of premature aging in HIV disease have focused on ART-naïve individuals, and have shown that naïve HIV-infected subjects share diverse immunological changes with elderly people. These similarities include a low naïve : memory T-cell ratio, expansion of cytomegalovirus (CMV)-specific CD8 T cells, higher percentages of CD57+CD27- ('senescent') and PD-1+ ('exhausted') T cells, increased CRP and interleukin-6 levels, reduced responses to vaccines, reduced T-cell telomere lengths and a low CD4:CD8 ratio [33-36]. However, the extent to which ART reverses these HIV-induced immune changes is currently the subject of ongoing investigation. Interestingly, most individuals (74%) in our study exhibited an inverted CD4:CD8 ratio despite at least 1 year of ART-mediated plasma viral suppression and CD4 T-cell count recovery. To the best of our knowledge, the clinical significance of this phenomenon in long-term ART-experienced subjects has not yet been investigated. Outside HIV infection, a low CD4:CD8 ratio, also termed the immune risk profile, is considered a surrogate marker of immunosenescence and has been shown to be an independent predictor of all-cause mortality [11, 37]. The immune risk profile is characterized by an increase in the number of CD8+CD28- cells, which results in a low CD4:CD8 ratio, and is associated with a high number of CMV-specific T cells or CMV seropositivity [9]. Similarly, in HIV infection, persistence of a low CD4:CD8 ratio despite CD4 T-cell count restoration may be explained by oligoclonal expansion of CD8 T cells, reflecting an underlying immunosenescence [9], raising the possibility that the CD4:CD8 ratio may also be a surrogate marker of immunosenescence in this population.
On the basis of this hypothesis, we recently explored the biological significance of the CD4:CD8 ratio in treated HIV-infected patients. In a cross-sectional analysis in 20 subjects with long-standing viral suppression, we observed that the CD4:CD8 ratio was an independent predictor of CD4 T-cell activation [13]. We also addressed the association between the CD4:CD8 ratio and T-cell activation in 37 vertically HIV-infected children and adolescents, finding that the inversion of the CD4:CD8 ratio was a strong independent predictor of higher percentages of CD8 T cells expressing activated (HLA-DR+CD38+), senescent (CD58+CD27-) and activated/exhausted (HLA-DR+PD-1+) phenotypes [14]. In view of the fact that immune activation is considered the major driving factor of this premature aging in HIV infection, these data suggested that inversion of the CD4:CD8 ratio might help to identify ART-treated patients at higher risk of noninfectious conditions. The present study provides new insight into the clinical significance of the CD4:CD8 ratio in treated HIV-infected subjects, as we have demonstrated its value as a variable independently associated with various aspects of the aging process: atherosclerosis, arterial stiffness and renal impairment.
Our study is subject to a number of limitations common to cross-sectional studies. In addition, we used surrogate markers of cardiovascular risk - IMT and arterial stiffness - instead of analysing cardiovascular events, which would have required a prospective study with a large sample size. Although IMT has been demonstrated to be an important predictor of cardiovascular events in the general population [20], and IMT measurement is the test recommended by the American Heart Association for the assessment of the atherosclerotic burden [21], a recent meta-analysis in the general population found no evidence of increased risk of cardiovascular events associated with faster IMT progression [38]; this recent study precludes us from drawing the conclusion that inversion of the CD4:CD8 ratio in treated HIV-infected patients may help to identify subjects at increased cardiovascular risk. Our sample was representative of a middle-aged, low-cardiovascular-risk population according to the Framingham risk score equation. Accordingly, we expected to have a low proportion of subjects with high IMT values (> 1 mm). As IMT correlates with the incidence of cardiovascular events and no threshold effect is observed [20], we considered use of the median IMT as an unbiased way to stratify subjects according to their degree of subclinical atherosclerosis. Similarly, we classified subjects according to their eGFR with a cut-off within the normal range (90-120 mL/min/1.73 m2). Of note, each 10 mL/min/1.73 m2 decrease in eGFR has been associated with a 20% increased OR of cardiovascular events in HIV-infected patients [39], and previously we have reported that incipient renal impairment is an independent marker of subclinical atherosclerosis in HIV-infected individuals [22]. Finally, only 22 women were included in this study (17%) and thus our results cannot be extrapolated to women.
All things considered, our data suggest that the CD4:CD8 ratio in HIV-infected subjects on effective ART is independently associated with surrogate markers of age-associated disease. Although the discriminative ability of the CD4:CD8 ratio as a marker of non-AIDS-related events must be confirmed in large prospective studies with clinical endpoints, we believe that the description of a surrogate marker of accelerated aging in successfully treated HIV-infected individuals may have important implications in both clinical and investigational settings. If the CD4:CD8 ratio is further validated as an independent predictor of non-AIDS-related morbidity, normalization of the CD4:CD8 ratio might be considered a clinical target. As the findings of two previous studies from our group also support the conclusion that the CD4:CD8 ratio is independently associated with immune activation [13, 14], and immune activation seems to drive non-infectious-disease-associated mortality and morbidity in treated HIV infection [5, 40], it may be of great interest to include subjects with a low CD4:CD8 ratio despite successful ART in clinical trials of interventions aiming to reduce chronic inflammation and immune activation. Also, treated individuals with the lowest CD4:CD8 ratios despite long-term viral suppression might be specially monitored for the prompt detection and early treatment of age-related conditions. It is noteworthy that the CD4:CD8 ratio is usually reported and available in routine clinical practice; thus, its use as a predictor of non-AIDS-related disorders might be easily implemented if more evidence is obtained to support our data.
In conclusion, in this study in treated HIV-infected subjects, a low CD4:CD8 ratio despite at least 1 year of effective ART was an independent marker of conditions associated with age-related disease - carotid atherosclerosis, arterial stiffness and renal impairment. These findings may have implications in both research and clinical settings.
|
|
| |
| |
|
|
|