| |
HIV-associated neurocognitive disorder
|
| |
| |
Download the PDF here
David B Clifford, Beau M Ances
Department of Neurology and Medicine (Prof D B Cliff ord MD) and Departments of Neurology, Bioengineering, and Radiology (B M Ances MD), Washington University in St Louis, St Louis, MO, USA
The Lancet Infectious Diseases November 2013
from jules: I remain convinced that HIV+ individuals can & often do experience at least asymptomatic or low-symptom cognitive dysfunction whether it is due to HIV itself or a constellation of potential causes that can include metabolic disorders which may be due in fact to HIV itself. But I remain convinced HIV itself does for at least some patients cause cognitive dysfunction that may or my not be obvious to patients. I also remain convinced HCV contributes to cognitive dysfunction in HCV mono and in coinfected patients, despite mixed study outcomes, and that coinfected can experience more cognitive or neurologic dysfunction since they have both diseases, when HCV is cured cognitive function usually improves increasingly over several years. Research studies outcomes are often slaves to the study design, study participants and the analyses & interpretation of these analyses subjecting them at times to not necessarily reflecting the reality of patient's experiences.
"A functional cure for HIV infection will need the virus to be silenced in all body compartments, including the brain......a silver lining of modern treatment is that more severe forms of neurocognitive impairment are rare, although milder forms remain. Establishment of the causes, prognosis, and optimum cART regimen for patients with HIV-associated neurocognitive disorder remains a major goal. An understanding of existing HIV-associated neurocognitive disorder definitions is essential.......Although the virus is recognised for its direct effect on the cellular immune system through depletion of infected CD4 lymphocytes, it also has broad effects on the nervous system, including evidence for direct pathology in the brain, spinal cord, and peripheral nerves........Far short of the quest for cure, progress towards elimination of HIV-associated neurological disability has been discouraging......The main issues remaining for neuroAIDS include the implications of persistent low levels of HIV, ongoing inflammatory responses, potential therapeutic toxicity, and interactions between ageing and neurodegeneration caused by the virus"
"Conclusions: Neurological involvement in HIV infection remains an important aspect of the infection that needs further research. Objective tests of neurological function confirm that cART, although improving outcomes immensely, has not accomplished full functional protection of the nervous system. Because changes are now subtle, and generally occur slowly, HIV-associated neurocognitive disorder remains challenging to study, but the importance of brain function to independence and quality of life demand that ongoing efforts are directed to optimise this aspect of care for HIV patients. Almost certainly, several mechanisms contribute, and therefore rational use of various therapeutic interventions will be needed to achieve success."
"the usefulness of the asymptomatic neurocognitive impairment category is controversial......In the Multicenter AIDS Cohort Study,29 investigators studied the neurocognitive performance of asymptomatic patients with HIV infection who were either immunologically intact or virologically controlled and HIV-seronegative people for 5 years. The patients with HIV infection had no decline on several neuropsychometric tests-an important and reassuring finding......However, some patients with HIV continue to experience neurocognitive deterioration despite virologically successful treatment.
Neurological function demands careful assessment, and in busy clinics, mild disorders might be overlooked. Longitudinal observations of a large US academic clinic cohort have been reported in preliminary form.30 Neurocognitive performance, laboratory measurements, and neurological examinations were done every 6 months for 42 months. Most (61%) patients with HIV remained stable, whereas 16·5% apparently improved neurocognitive status and 22·7% declined.30 Factors associated with risk of progression included the presence of severe comorbidities (eg, other infections, drug abuse, or other neurological disorders) or evidence of HIV treatment failure (off ART, low CD4 cell count, or both). A limitation of this study is that appropriate HIV-seronegative control participants were not included.......Within the AIDS Clinical Trials Group, in one of the longest clinical trials that monitored patients on cART, no substantial decline in neurocognitive performance was seen during 3 years of observation.34"
Summary
Neurological involvement in HIV is often associated with cognitive impairment. Although severe and progressive neurocognitive impairment has become rare in HIV clinics in the era of potent antiretroviral therapy, most patients with HIV worldwide have poor outcomes on formal neurocognitive tests. In this Review, we describe the manifestations of HIV-associated neurocognitive disorder in the era of effective HIV therapy, outline diagnosis and treatment recommendations, and explore the research questions that remain. Although comorbid disorders, such as hepatitis C infection or epilepsy, might cause some impairment, their prevalence is insufficient to explain the frequency with which it is encountered. HIV disease markers, such as viral load and CD4 cell counts, are not strongly associated with ongoing impairment on treatment, whereas cardiovascular disease markers and inflammatory markers are. New cerebrospinal fluid and neuroimaging biomarkers are needed to detect and follow impairment. Ongoing research efforts to optimise HIV therapy within the CNS, and potentially to intervene in downstream mechanisms of neurotoxicity, remain important avenues for future investigation. Ultimately, the full control of virus in the brain is a necessary step in the goal of HIV eradication.
Introduction
HIV emerged as a major threat to world health over 30 years ago, and has challenged scientists and clinicians to combat its vast and devastating effects. Although the virus is recognised for its direct effect on the cellular immune system through depletion of infected CD4 lymphocytes, it also has broad effects on the nervous system, including evidence for direct pathology in the brain, spinal cord, and peripheral nerves.1 This primary HIV-associated neurocognitive disorder, combined with a unique range of opportunistic infections and malignant disease, constitutes neuroAIDS.
Development of combined antiretroviral therapy (cART) has changed HIV to a chronic disease with a life expectancy approaching population norms for patients who comply with treatment.2 The main issues remaining for neuroAIDS include the implications of persistent low levels of HIV, ongoing inflammatory responses, potential therapeutic toxicity, and interactions between ageing and neurodegeneration caused by the virus.3-6 A functional cure for HIV infection will need the virus to be silenced in all body compartments, including the brain.7
HIV is more prevalent in developing countries than in developed countries. Because treatment is often delayed, a heavy disease burden persists in these settings because of neurological opportunistic infections, especially cryptococcal and tubercular meningitis, toxoplasma encephalitis, and progressive multifocal leukoencephalopathy.8, 9 These complications mostly stop after stable cART has been achieved. Additionally, peripheral neuropathy can affect the quality of life of patients with HIV, but is reduced when treatment with non-neurotoxic antiretrovirals is started early after infection.10 These important neuroAIDS topics are reviewed elsewhere.8-10 We restrict this Review to HIV-associated neurocognitive disorder.3-5
Far short of the quest for cure, progress towards elimination of HIV-associated neurological disability has been discouraging.11, 12 Cross-sectional studies continue to show that about half of all treated patients with HIV have cognitive impairment, which represents little improvement compared with the pre-cART era.
However, a silver lining of modern treatment is that more severe forms of neurocognitive impairment are rare, although milder forms remain. Establishment of the causes, prognosis, and optimum cART regimen for patients with HIV-associated neurocognitive disorder remains a major goal. An understanding of existing HIV-associated neurocognitive disorder definitions is essential. A consensus research definition13 of HIV-associated neurocognitive disorder includes the subclassifications asymptomatic neurocognitive impairment, mild neurocognitive disorder, and HIV-associated dementia (table).
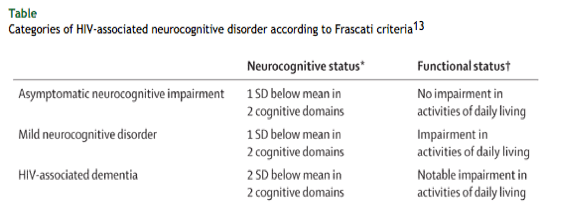
SD=standard deviation.
Neurocognitive testing should include assessment of at least five domains, including attention-information processing, language, abstraction-executive, complex perceptual motor skills, memory (including learning and recall), simple motor skills, or sensory perceptual skills. Appropriate norms must be available to establish the number of domains in which performance is below 1 SD.
Functional status is typically assessed by self-reporting but might be corroborated by a collateral source. No agreed measures exist for HIV-associated neurocognitive disorder criteria. Of note, for diagnosis of HIV-associated neurocognitive disorder, other causes of dementia must be ruled out and potential confounding effects of substance use or psychiatric illness should be considered.
Key messages
· Neurocognitive ability is impaired in most patients with HIV
· Severe dementia rarely develops in patients on effective combined antiretroviral therapy
· Most patients with mild neurocognitive impairment are clinically stable
· Comorbid disorders contribute to neurocognitive impairment but do not fully explain it
· Typical HIV disease biomarkers (viral load or CD4) are no longer closely associated with impairment
· Cardiovascular disease and inflammatory markers are associated with impairment
· Neuroimaging and cerebrospinal fluid studies could provide new mechanisms to improve our understanding of HIV-associated neurocognitive disorder
· Optimum HIV therapy is necessary, but not sufficient, to avert cognitive impairment
· Neither higher CNS-penetrating combined antiretroviral therapy nor adjuvant treatments have proven to be effective to reverse HIV-associated neurocognitive disorder
· Treatment to address inflammation and cardiovascular risks seems to be a rational approach
Clinical manifestations
HIV-associated dementia
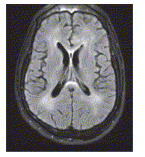
Independent of opportunistic diseases, advanced HIV infection is associated with cognitive impairment-the consequence of HIV infection within the nervous system.14 AIDS dementia complex, a subcortical dementia, was characterised as a progressive disabling disorder that manifested as an increase in loss of attention and concentration, notable motor slowing, and various behavioural components, and generally led to death within 1 year.15 This syndrome was associated with pathological changes in the brain that include generalised atrophy, changes in white matter causing leukoencephalopathy (figure), microglial nodules typical of viral encephalitis, and multinucleated giant cells that seem to be infected directly by HIV on antigen staining.16, 17 In untreated infection, severity of dementia was more closely associated with inflammatory response markers than with viral load, although cerebrospinal fluid (CSF) viral load was modestly associated with clinical manifestations.18-24 The progressive impairment described as AIDS dementia complex is now referred to as HIV-associated dementia, according to recent criteria.13
Characteristic MRI findings of a patient with HIV-associated neurocognitive disorder
A FLAIR (fluid level attenuated inversion recovery image) was obtained and shows prominent white matter changes throughout the brain. These changes are typically seen only in more advanced disease and are rare in the post-combination antiretroviral therapy era.
------------------------------
However, confusion could still persist because the term HIV-associated dementia is used not only for progressive brain disease caused by untreated AIDS, but also for substantial residual HIV-associated neurological impairment. Not unexpectedly, the substantial and growing population who have incurred brain injury because of HIV, but do not have obvious progressive disease, will not benefit from the same treatments as those with active virally mediated pathology. Extensive research in neuroAIDS has not been able to separate these populations, which contributes to disappointing or confusing assessments. Application of existing HIV-associated neurocognitive disorder criteria has relied too heavily on diagnosis based on the severity of neurological impairment on neuropsychological examination. The dynamic status of impairment and the associated pathophysiology should be included in diagnostic and therapeutic efforts, which could help the specialty to address the remaining issues more effectively.
Mild neurocognitive disease and asymptomatic neurocognitive impairment
The prevalence of HIV-associated neurocognitive disorder is driven by asymptomatic neurocognitive impairment and mild neurocognitive disorder (table). Compared with the best available population norms, about half of patients with HIV infection continue to have performance levels lower than expected.11, 12, 25 However, existing HIV-associated neurocognitive disorder research definitions for asymptomatic neurocognitive impairment and mild neurocognitive disorder might be too inclusive and lead to clinically unimportant inflation of impairment data.26 Based on neuropsychometric performance that lacks ideally matched norms, knowledge of previous performance, and exclusion of many confounding issues, the usefulness of the asymptomatic neurocognitive impairment category is controversial.
The definition of functional impairment-the feature that distinguishes mild neurocognitive disorder from asymptomatic neurocognitive impairment-further confounds existing criteria. In practice, assessment of functional impairment is challenging and probably imprecise. A range of approaches can be used to define functional capacity. Typically, self-report has been used but is a subjective approach. If functional impairment is recorded, to distinguish whether changes are due to HIV infection or other causes is often difficult. Formal scales for functional impairment (ie, the Lawton Brody scale27) were developed for other neurodegenerative disorders and are rather dated. More quantifiable performance measures have been developed that might be more appropriate for patients with HIV infection (eg, the Columbia medication management test and the San Diego finances test28). However, self-report and performance-based measures are linked to educational, cultural, and societal biases and cannot predict who will develop progressive impairment. Moreover, discrimination between asymptomatic neurocognitive impairment and mild neurocognitive disorder remains difficult because the imprecision of these categories means that patients might fluctuate between these two states. Although this finding could be due to biological factors, limitations of definitions of disorders as used in complex patients over time and testing imprecision could be equally likely to contribute to this uncertainty. Long-term trajectories of performance are urgently needed to better understand the potential prognostic implications of these categories.
Longitudinal observations
In the Multicenter AIDS Cohort Study,29 investigators studied the neurocognitive performance of asymptomatic patients with HIV infection who were either immunologically intact or virologically controlled and HIV-seronegative people for 5 years. The patients with HIV infection had no decline on several neuropsychometric tests-an important and reassuring finding. The tests were sensitive enough to detect the expected age-related decline, but did not show a greater deterioration in the asymptomatic HIV-positive population than in HIV-seronegative controls enrolled in the same study, even in patients with imperfect viral control. This finding agrees with our impression in the clinics and has been shared by many HIV care providers who report little evidence of a widespread deterioration of neurocognitive performance in excess of that attributable to ageing or comorbidities.
However, some patients with HIV continue to experience neurocognitive deterioration despite virologically successful treatment.Neurological function demands careful assessment, and in busy clinics, mild disorders might be overlooked. Longitudinal observations of a large US academic clinic cohort have been reported in preliminary form.30 Neurocognitive performance, laboratory measurements, and neurological examinations were done every 6 months for 42 months. Most (61%) patients with HIV remained stable, whereas 16·5% apparently improved neurocognitive status and 22·7% declined.30 Factors associated with risk of progression included the presence of severe comorbidities (eg, other infections, drug abuse, or other neurological disorders) or evidence of HIV treatment failure (off ART, low CD4 cell count, or both). A limitation of this study is that appropriate HIV-seronegative control participants were not included.
Results of clinical trials of patients with HIV-associated neurocognitive disorder have reinforced the impression that cognitive changes due to HIV, if present, must be slow, since they do not typically occur in control groups in trials of short duration.31-36 Within the AIDS Clinical Trials Group, in one of the longest clinical trials that monitored patients on cART, no substantial decline in neurocognitive performance was seen during 3 years of observation.34
Clinical presentation
Clinical neuropsychological manifestations of HIV-associated neurocognitive disorder in the cART era differ substantially from the classic descriptions of AIDS dementia complex.15 In the pre-cART era, a progressive subcortical dementia with motor and cognitive slowing was prominent. In the earliest clinical trials, as treatment was initiated, patients showed reliable improvement on timed motor tasks and obvious clinical neurological progress.37, 38 However, in the cART era, more cortical than subcortical involvement is often recorded.25 A comparative analysis assessing patients with HIV and HIV-seronegative patients from the pre-cART and post-cART eras has shown that in people with HIV, impairments in motor skills, cognitive speed, and verbal fluency predominated in the pre-cART era, whereas impairments in memory (learning) and executive function predominated in the post-cART era.25 Subtle cognitive changes in HIV patients in the post-cART era are also seen in prospective memory, or the ability to "remember to remember",39, 40 which could affect function in the workplace and cause problems with adherence to medication. Tests of prospective memory need to be done in the clinical setting because patients might be unaware of their deficits.41 Notably, the pattern of cognitive dysfunction now reported with HIV-associated neurocognitive disorder is more similar to other more common degenerative disorders (ie, Alzheimer's disease) than to classic HIV-associated dementia, which could create challenges in the differentiation of these diseases in elderly patients with HIV.
Assessment of the disorder
Neuropsychometric performance testing
NeuroAIDS research has relied heavily on neuropsychometric performance testing for both diagnosis and monitoring. This approach worked well in the pre-cART era when obvious progressive HIV-associated dementia could be identified, and when successful intervention with zidovudine monotherapy resulted in unequivocal benefits on neurocognitive tests.38, 42 However, in the cART era, use of neuropsychometric performance testing is more challenging since the major aim is to identify patients with more subtle deficits. Detection of mild deficits needs more difficult tests that often take longer than the simple timed motor tests of the pre-cART era. Identification of short and tolerable screening tests appropriate for busy HIV clinics in both high-income and low-income countries has been difficult.41,43-45 Frequently used methods include versions of the mini-mental status exam, the Montreal cognitive assessment, the composite Z scores of several brief neuropsychometric tests used in the AIDS Clinical Trials Group system, the International HIV dementia screen, and parts of CogState devices. These tests are generally not ideal because they are neither sensitive nor specific for HIV-associated neurocognitive disorder.41,44,46-48 Although consensus suggests that neuropsychometric performance should be measured repeatedly (at least in research), no agreement exists regarding the exact battery to use.49 To encourage routine repeated implementation of neurocognitive screens in clinic patients might be premature. Neurocognitive testing will continue to have a valuable role in longitudinal assessments for research, but additional reliable biomarkers with more pathophysiological validity are needed to transform this specialty.
Neuropsychometric testing should continue to be used to assess the efficacy of interventions for use in therapeutic trials. When patients are their own controls and are compared with other patients monitored similarly by the same investigators over time, these measures can be sensitive for detection of change relevant to therapeutic interventions. Comparative groups studied with the same sequence of testing can account for the learning effects, which might be difficult to detect without concurrent controls. Increased knowledge about accounting for the effect of practice and familiarity with repeat testing and of the clinical implications of test performance by study of so-called meaningful change could further enhance the interpretation of more widely available observational data.50 However, particular caution should be used when these tests are used internationally and across social and cultural groups. Normative data are greatly affected by highly complex characteristics of administration and the tested population. Careful recruitment of appropriate controls is necessary. Because HIV is more prevalent in developing than in developed countries, and because neuropsychometric tests are substantially affected by social and economic factors, great effort should be made to develop appropriate local normative assessments. Ideally, these norms would be gathered in parallel with active data collection for research at low-income sites. However, practical considerations have led to less robust norms for low-income sites than are available for US and European cohorts. Other pathophysiological biomarkers are therefore of especially great importance in assessment of disease manifestations in developing countries.
Systemic and plasma markers
Targeting of full systemic control of HIV hardly needs justification based on neurological consequences, since most clinicians now strongly ascribe to the benefits of early and complete control of the virus. The preponderance of evidence about HIV-associated neurocognitive disorder reinforces the primary importance of systemic control of HIV to achieve optimum neurological outcomes. However, in the cART era, HIV load and CD4 cell counts are no longer closely associated with neuropsychometric performance.51, 52 Studies suggest that a low CD4 nadir increases the risk for HIV-associated neurocognitive disorder, whereas a recent study of military patients shows that early treatment could substantially prevent the disorder.25,53-57
Additional blood markers that are associated with HIV-associated neurocognitive disorder or that could identify a population at risk of neurological progression have been difficult to isolate. One of the more promising areas for potential peripheral biomarkers has been monitoring of monocyte activation. Within the brain, monocytes and macrophages seem to be important cells that not only carry the virus, but can also release potentially deleterious cytokines when activated. Contributions to this inflammatory response can originate outside the brain and subsequently invade it. Plasma-soluble CD14 is a potential biomarker, which has been linked to impairment in attention and learning in patients with HIV-associated neurocognitive disorder.58 Detection of HIV DNA circulating within mononuclear cells could also identify a raised risk for HIV-associated neurocognitive disorder.59-62 These results are consistent with the idea of increased trafficking of activated monocytes to the brain, which can lead to neurocognitive impairment.58, 63, 64 Another measure of activated monocytes seems to be soluble CD163-a scavenger receptor that is upregulated in activated monocytes. Recent studies suggest that this receptor might remain raised in patients with HIV-associated neurocognitive disorder on stable cART, which might allow the selection of such patients whose disease is driven by ongoing systemic cellular activation.65, 66
Peripheral inflammatory disease from several potential sources might be relevant to HIV-associated neurocognitive disorder. HIV affects the gut to potentially cause microbial translocation driving chronic inflammation, leading to HIV-associated dementia.67, 68 Strategies to reduce such activation might yield neurocognitive benefits. Cardiovascular risk markers, which can also be driven by chronic immune activation, have been associated with HIV-associated neurocognitive disorder and provide a potentially crucial modifiable factor. In the Multicenter AIDS Cohort Study, carotid intima-media thickness and glomerular filtration rate were associated with performance speed on neuropsychometric tests, and intima-media thickness was also associated with memory impairment.51 In the Strategies for Management of Antiretroviral Therapy study, cardiovascular risks, hypertension, and hypercholesterolaemia were more closely associated with baseline neuropsychometric performance results than were HIV disease markers.52 The more recent CNS HIV Anti-Retroviral Therapy Effects Research study has also shown that the increased presence of metabolic risk factors was linked to HIV-associated neurocognitive disorder.69
CSF markers
Assessments of the CSF represent the biology of the CNS more closely than does blood. The blood-brain barrier limits movement from blood to brain compartments, to create a physiological compartment. Patients and physicians are often unduly reluctant to do a lumbar puncture for CSF collection. In the absence of abnormal clotting or a large asymmetric mass in the brain, lumbar punctures are exceedingly safe and, when done by skilled clinicians, are not as harmful as are many other procedures. Use of non-cutting needles greatly reduces the chance of transient orthostatic headaches that sometimes follow lumbar puncture. When unique information can be collected by a lumbar puncture, the procedure should be done.
Occasionally HIV emerges in the CSF heralded by new neurological symptoms or signs, even in the face of continued blood virological control.70-72 This phenomenon of viral escape is uncommon but should alert the clinician to assess the CSF if new or active neurological symptoms are present that are not otherwise explained. Rarely, asymptomatic patients also have detectable HIV in low copy numbers when blood virus concentrations are well controlled.73-75 Little evidence exists for CSF viral evolution in successfully treated HIV patients, but further longitudinal CSF studies of treated patients are needed.
The characteristics of virus recovered from CSF or from the CNS might represent the unique characteristics of a neurotropic virus.76 Interest in changes in tat sequences across viruses has raised the possibility that variation in clade neurotropism might relate to tat sequence differences.77-79 Mutations can occur at specific sequences of the viral genome and can affect the ability of the virus to successfully bind to and enter macrophages.80-82 The HIV epidemic has resulted in several subtypes of HIV evolving worldwide and the possibility that these also diverge in neurological manifestations has been an area of active research. Differing prevalence of HIV-associated dementia between subtypes A and D in Uganda suggested varying pathogenicity,83 but overall comparisons of different HIV clades with neuropsychometric performance have mainly shown similar results across regions.84 Little evidence exists to connect specific viral characteristics to the ultimate development of milder HIV-associated neurocognitive disorder. HIV RNA and DNA in the brain at post mortem are most strongly associated with multinucleate cell encephalitis with HIV-associated dementia. Pathological findings and viral recovery are not associated with mild forms of HIV-associated neurocognitive disorder. Notably, asymptomatic neurocognitive impairment and mild neurocognitive disorder do not have specific neuropathological correlates.85, 86
The clinical usefulness of inflammatory markers in the CSF has been low, but these measures could potentially identify patients at risk of HIV-associated neurocognitive disorder. Even patients on long-term suppressive cART have mildly raised CSF neopterin and IgG index.87 Persistent immune activation markers, including interleukin 6, interleukin 8, and CCL2 (MCP-1), remain present in successfully treated cART patients.88Another situation in which dysregulated immune response can drive impairment and symptoms is during immune reconstitution, especially in the rare but dramatic CD8 encephalitis cases reported during treated HIV infection.89-91
Markers of neuronal injury could also be associated with more advanced cognitive impairment.92-96 In particular, the concentration of neurofilament light protein is raised in untreated patients with HIV-associated neurocognitive disorder and decreases with successful treatment. Concentration of tau protein might be raised in HIV-associated dementia but not in patients with asymptomatic neurocognitive impairment or mild neurocognitive disorder, although data remain inconsistent for this potential biomarker.95-98
Neuroimaging
Neuroimaging techniques are continuing to develop and might have increased usefulness in the diagnosis and management of HIV-associated neurocognitive disorder. Metabolic, structural, and functional modalities have been used in research settings. Many of these techniques hold great promise because they can be easily added to conventional scans that are often obtained from patients with HIV. Metabolic imaging with magnetic resonance spectroscopy has been done in both the pre-cART and post-cART eras.99-102 These studies measure metabolite ratios that are indicative of either neuronal function (N-acetylaspartate) or inflammation (choline or myoinositol) compared with a reference marker (creatine). In the pre-cART era, decreases in the ratio of N-acetylaspartate to creatine and increases in the ratio of choline to creatine were recorded with HIV infection.103 However, these measures might be normalised after cART administration.104 Additionally, this technique might be limited because only particular brain regions or voxels are studied. Other metabolic studies, such as PET, have mainly been done in the pre-cART era. A biphasic response was recorded, with early increases followed by progressive declines with more advanced cognitive impairment.105 Large studies with PET imaging in the post-cART era are needed. More recently, PET imaging with ligands specific for microglial activation have been investigated, with the aim of quantifying and localising brain inflammation.106-108
Two structural neuroimaging techniques have been of particular interest: volumetric analysis and diffusion tensor imaging. Structural neuroimaging reveals changes not only within subcortical areas but also cortical areas.109-112 These changes can accrue early after seroconversion and persist even after cART is started.113 Diffusion tensor imaging can measure diffusion of water molecules in white matter tracts, specifically within the corpus callosum-a white matter tract connecting the two hemispheres. A decrease in mean diffusivity and an increase in fractional anisotropy have typically been associated with HIV infection. These measures can improve after initiation of cART114-117 and show substantial promise.
More recently, functional neuroimaging measures have been employed that use MRI for blood oxygen concentration-dependent imaging and arterial spin labelling. For straightforward functional tasks, increased recruitment of additional areas might occur in patients with HIV to meet cognitive demands.118, 119 When assessed at rest, functional connections between brain networks can be compromised in HIV-associated neurocognitive disorder, in ways that are similar to ageing.120 Arterial spin labelling allows for non-invasive measurement of cerebral blood flow, which is a time-linked measure of brain metabolism. A decrease in cerebral blood flow has been reported soon after seroconversion, with HIV-positive patients having cerebral blood flow values equivalent to HIV-seronegative people who are 15-20 years older.121 Administration of cART can lead to improvements in cerebral blood flow, but the values do not normalise completely.121, 122 These results suggest that this technique could be a good measure to assess the efficacy of new treatments. With the development of new methods, multicentre trials that use common neuroimaging sequences are needed.
Antiretroviral therapy
The introduction of cART has had clear benefits. As a result of immune reconstitution, opportunistic disorders have become rare, and HIV-associated dementia now develops infrequently. However, variability exists in the penetration and transport of antiretroviral drugs across the blood-brain barrier, which has led to serious concerns about the brain acting as a nidus where virus persists under partial control. In theory, resistant virus could evolve in the CNS and reseed the body. Clearance of the virus from the CNS is necessary to achieve a cure for HIV infection. The practical evidence of long-term successful suppression of HIV in the cART era with only rare CNS escape reassures us that almost all drug combinations control the virus in the CNS compartment. However, this theoretical risk, along with recognition of ongoing HIV-associated dementia, has appropriately inspired intensive consideration and monitoring of treatment. One explanation for the continued prevalence of HIV-associated neurocognitive disorder is that low-level viraemia in the CNS can continue, and drives neurodegeneration either by toxic inflammatory activation or toxic viral products such as the tat protein.3
The notion of CNS penetration effectiveness of drug regimens was formalised to encourage study of the association between the efficacy of antiretrovirals and their ability to enter and function in the CNS. A ranking scale123 was created on the basis of available information about existing antiretrovirals, including their physicochemical properties, known CSF drug concentrations, and effectiveness at virus clearance in the CSF. Each drug in a regimen was given a score for relative effectiveness, and the scores were summed for the regimen overall.123 The model seems to have validity, supported by some findings, that CSF viral loads are more likely to remain controlled when the CNS penetration effectiveness is high.123 However, the effect seems to be inconsistent across studies, and is imperfectly linked to the degree of cognitive impairment or survival in treatment studies.35,56,124-126 A prospective test of the validity of CPE was reported recently.127 In a randomised trial,127 investigators compared treatment of patients with mild-to-moderate HIV-associated neurocognitive disorder, with participants randomly assigned to receive regimens with either high or low CNS penetration effectiveness that were otherwise expected to be active against the virus. Accrual was challenging, and eventually the study was stopped short of study goals. However, with 49 evaluable patients included and randomly allocated, no significant neuropsychometric differences between regimens were recorded. Presently, there seems to be no reason to use this strategy to select cART routinely. However, continued research into the variable effectiveness of treatment should be done. As more information is acquired about drug activity and distribution, ongoing revisions of CNS penetration effectiveness might enhance its power. If viral breakthrough in the CNS is discovered, viral drug sensitivity should be tested and treatment should be changed to the most potent, tolerable, and straightforward regimen available for that isolate. CNS penetration effectiveness might also provide information about the possibility of drug toxicity. Although unproven, in vitro and some in vivo observations are consistent with chronic drug toxicity contributing to neurological impairment.35, 128, 129
An alternative therapeutic strategy designed to address neurocognitive impairment has indicated that monocytes and macrophages seem to be the primary cellular reservoir affecting the CNS, representing resident cells with proliferative infection in the untreated brain that potentially harbour the virus in circulating monocytes, even during effective treatment.60 When therapy was graded by effectiveness for monocyte infection, cognitive outcomes seemed to be associated with this index. This strategy deserves further investigation.62, 130
The demonstration of ongoing viral breakthrough in some CSF samples, and the recognition that very low levels of virus are detected if ultrasensitive assays are used, has also spurred consideration of treatment intensification regimens that include newer classes of drugs. CCR5 antagonists are predicted to contribute uniquely to CNS isolates, whereas integrase inhibitors deserve further assessment for potency in the CNS. Although results of small intensification trials have been disappointing so far, larger multicentre trials could better address this possibility.131, 132Application of nanoparticles to increase delivery of antiretrovirals to the brain is also under investigation, with the caveat that more effective delivery might also increase intrinsic toxicity.133
Adjuvant therapy
With the recognition that HIV in the brain is necessary but not sufficient for manifestations of HIV-associated neurocognitive disorder, application of adjuvant therapies based on potential downstream pathological mechanisms has also been considered.134 Most of the hypotheses tested are derived from in-vitro findings or animal model results. So far, despite many phase 2 trials in human beings, none have provided convincing evidence for significant therapeutic benefit when active versus inactive adjuvant therapy groups were compared. In a recent trial, investigators tested minocycline, which is thought to inhibit microglial activation and to have antioxidative and neuroprotective properties. In a simian immunodeficiency virus model of encephalitis, minocycline reduced brain inflammatory disease.135 No benefit in neurocognitive status or in disease markers was recorded in antiretroviral-treated36 or untreated136 populations with HIV-associated neurocognitive disorder. An ongoing trial is investigating fluconazole and a selective serotonin reuptake inhibitor based on animal models of HIV-associated neurocognitive disorder and cohort data associated with selective serotonin reuptake inhibitors.137 Use of drugs approved for Alzheimer's disease has been considered. Memantine, an NMDA antagonist with neuroprotective properties in vitro, did not lead to neuroprotection or cognitive improvement, whereas in a recent small trial, an acetyl cholinesterase inhibitor seemed to provide slight symptomatic improvement.138-141 Valproic acid is of interest for HIV, most recently as a histone deacetylase inhibitor that might be used for cellular activation in a cure strategy.142, 143 It also seems to have neuroprotective properties.144, 145 A small study showed a trend towards cognitive improvement with neuropsychometric tests and neuroimaging measures.146 However, this finding was not substantiated in another trial.147 Anti-inflammatory strategies are the most often discussed adjuvant therapy for HIV-associated neurocognitive disorder. Forthcoming assessments of low-dose methotrexate, and large trials evaluating statins, might provide opportunities to further investigate the effect of inflammation on neurocognitive function.
Ageing population
Research into special interactions between anticipated changes of ageing and chronic HIV infection have attracted attention, most prominently surrounding the possible interactions of immunosenescence.148 Whereas HIV-associated dementia in the pre-cART era was characteristic of subcortical dementia (unlike Alzheimer's disease), now the clinical presentation of HIV-associated neurocognitive disorder can be similar to that of Alzheimer's disease. HIV neurocognitive impairment might, therefore, result from earlier expression of degenerative brain disorders such as Alzheimer's disease driven by HIV. Some reports support that pathological findings are consistent with Alzheimer's disease, but a fully consistent pathological confirmation has not been described.149, 150 Some reports suggest at least a partial overlap between CSF biomarkers for Alzheimer's disease and HIV-associated neurocognitive disorder. However, differences in CSF biomarkers do exist between the two neurodegenerative disorders.98, 151, 152 Years before the onset of Alzheimer's disease, changes in amyloid metabolism in the brain are indicated by amyloid plaque deposition that can be detected with positron imaging markers.153 Amyloid imaging scans have also been done in HIV-positive patients with no increases in amyloid deposition seen in patients with HIV-associated neurocognitive disorder.154 Furthermore, a genetic predisposition for Alzheimer's disease has been reported with the APOE 4 allele. Within the CHARTER study the presence of at least one APOE 4 allele was not associated with increased incidence of HIV-associated neurocognitive disorder.155 However, previous studies have shown some effect of APOE 4, generally in advanced disease or elderly patients.155-160 Ongoing attention to interactions of HIV with ageing processes, especially in the brain, will continue to be an important focus of research as the HIV population ages.148
Conclusions
Neurological involvement in HIV infection remains an important aspect of the infection that needs further research. Objective tests of neurological function confirm that cART, although improving outcomes immensely, has not accomplished full functional protection of the nervous system. Because changes are now subtle, and generally occur slowly, HIV-associated neurocognitive disorder remains challenging to study, but the importance of brain function to independence and quality of life demand that ongoing efforts are directed to optimise this aspect of care for HIV patients. Almost certainly, several mechanisms contribute, and therefore rational use of various therapeutic interventions will be needed to achieve success.
|
|
| |
| |
|
|
|