| |
Anti-PD-L1 immunotherapy in ARV-suppressed rhesus monkeys
|
| |
| |
6th Intl Workshop on HIV Persistance during Therapy Report Summary - Report by David Margolis MD, UNC Chapel Hill and the Collaboratory of AIDS Researchers for Eradication (CARE) - (12/15/13)
Reported by Jules Levin
6th HIV Persistence Workshop
Miami, December 5, 2013
James Whitney
Center for Virology and Vaccine Research, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA
PROGRAM ABSTRACT:
Anti-PD-L1 Immunotherapy in ARV-suppressed Rhesus Monkeys
J Whitney1, S Sanisetty1, Christa Osuna- Gutierrez1, S Lim1, Scott Balsitis2, Susan Chaniewski2, Volodomyr Gali2, Stephen Mason22Center for Virology and Vaccine Research, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston,
BACKGROUND: The testing of BMS-936559 presently is underway in antiretroviral-suppressed SIVmac251-infected rhesus macaques. The primary endpoint of this study is to assess safety during repeated I.V. administration of BMS-936559, and any improvement to the function of SIV-specific T cells. Our secondary endpoint is to evaluate perturbations in the latent reservoir and viral recrudescence after cessation of ARV therapy.
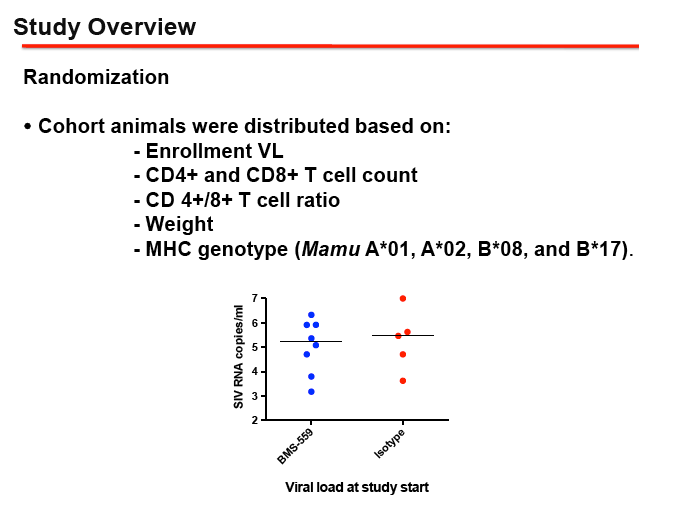
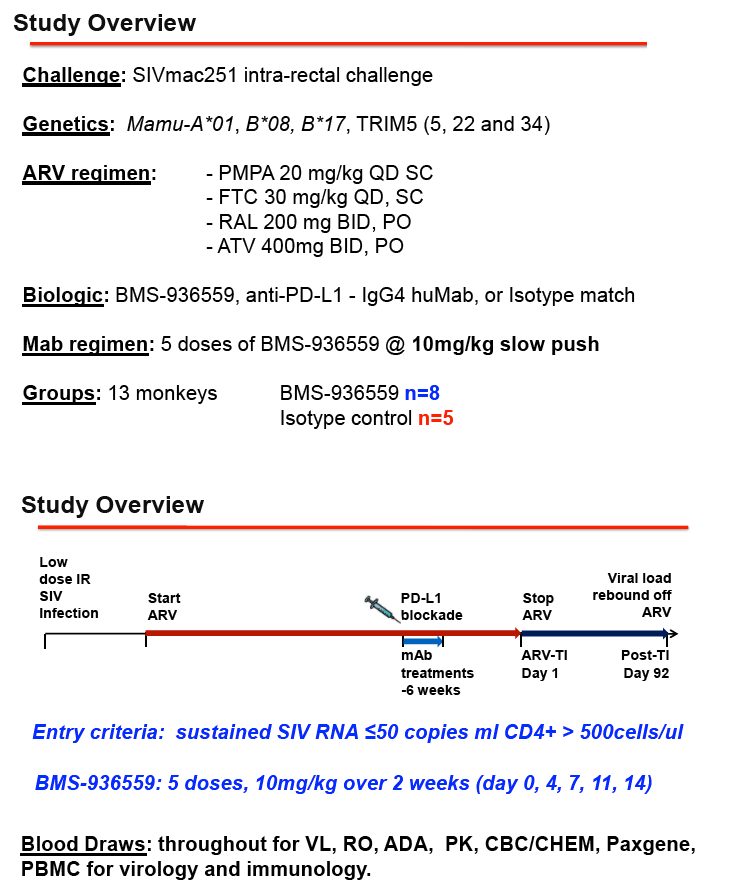
METHODS: 13 MHC-defined rhesus macaques were confirmed SIVmac251 positive and ARV treatment was initiated, using a 4 drug ARV regimen, for a minimum of 6 months prior to the administration of BMS-936559. All 13 animals received either BMS-936559 (N=8) or isotype control antibody (N=5). Multiple immunologic and virology analyses were used to evaluate ARV regimen efficacy, BMS-936559 dosing safety, BMS-936559 receptor occupancy. Any perturbations in the SIV reservoir in both tissues and blood will also be examined.
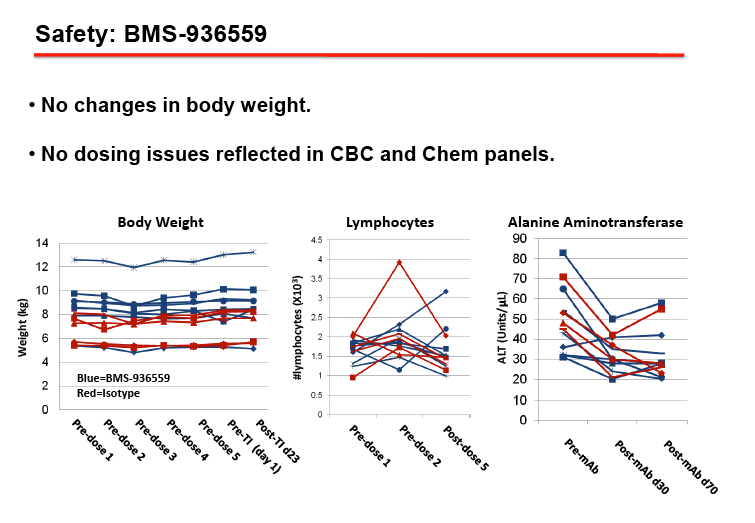
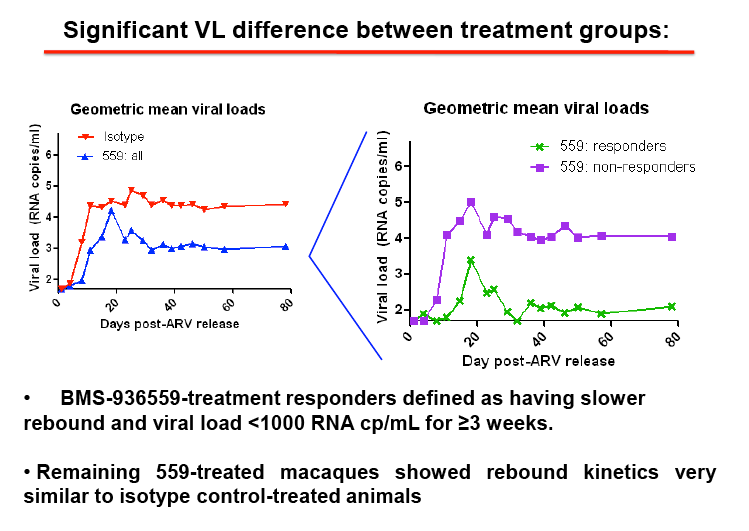
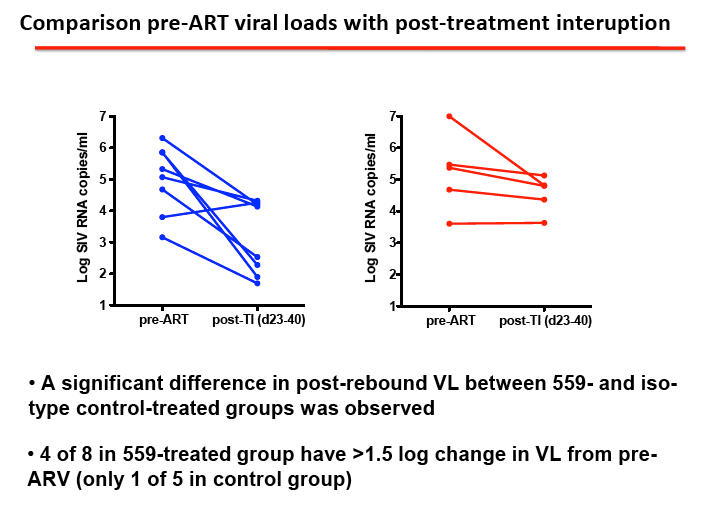
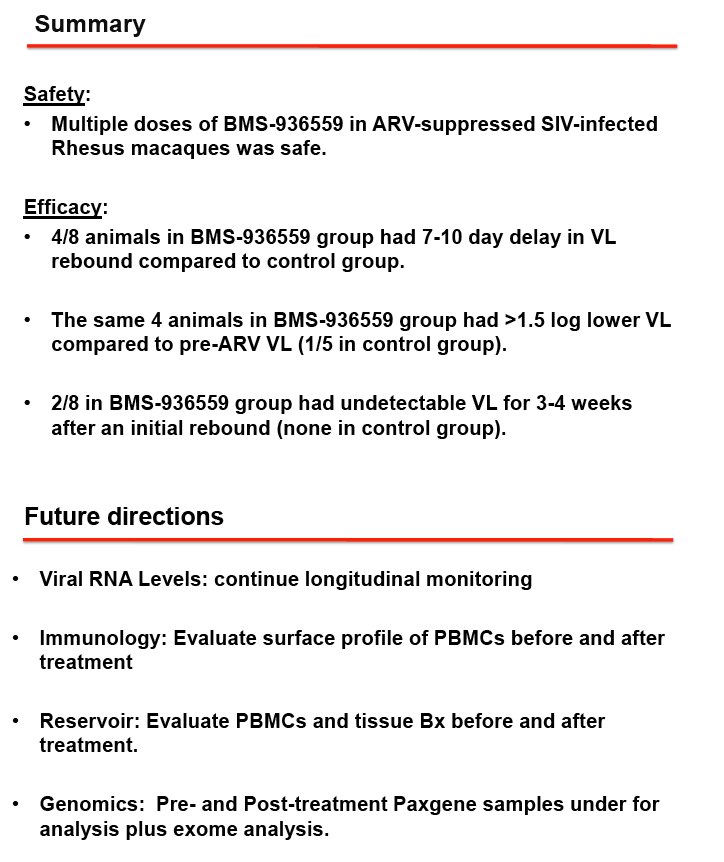
RESULTS: We observed durable virologic suppression of SIVmac251 at or below 50 RNA copies/ml in all animals with the application of our well-tolerated, 4-drug regimen. Significant reductions in inflammation markers were observed post ARV treatment. The repeated dosing of BMS-936559 was also well tolerated in all animals with no noted untoward effects. Receptor occupancy of BMS-936559 was favorable. Perturbations to proviral DNA levels (post-BMS-936559 treatment) and SIV viral RNA levels were observed after ARV release.
CONCLUSIONS: Combined, these data provide proof of concept for the safety and the repeated high-level dosing of BMS-936559 in vivo.
|
|
| |
| |
|
|
|