 |
 |
 |
| |
Low-Dose Lopinavir/Ritonavir Tablet Noninferior to Full Dose in Thai Children
|
| |
| |
7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur
Mark Mascolini
A lopinavir/ritonavir maintenance dose 70% of the FDA-recommended dose proved noninferior to the full dose in keeping viral load below 50 copies for 48 weeks in a multicenter Thai study [1]. Children had already reached a viral load below 50 copies with an initial full-dose protease inhibitor combination.
Notably, though, children weighing 35 to 50 kg had more than tripled odds of virologic failure compared with children weighing less. And twice as many children taking the low dose had a lopinavir trough below 1 mg/dL at week 48.
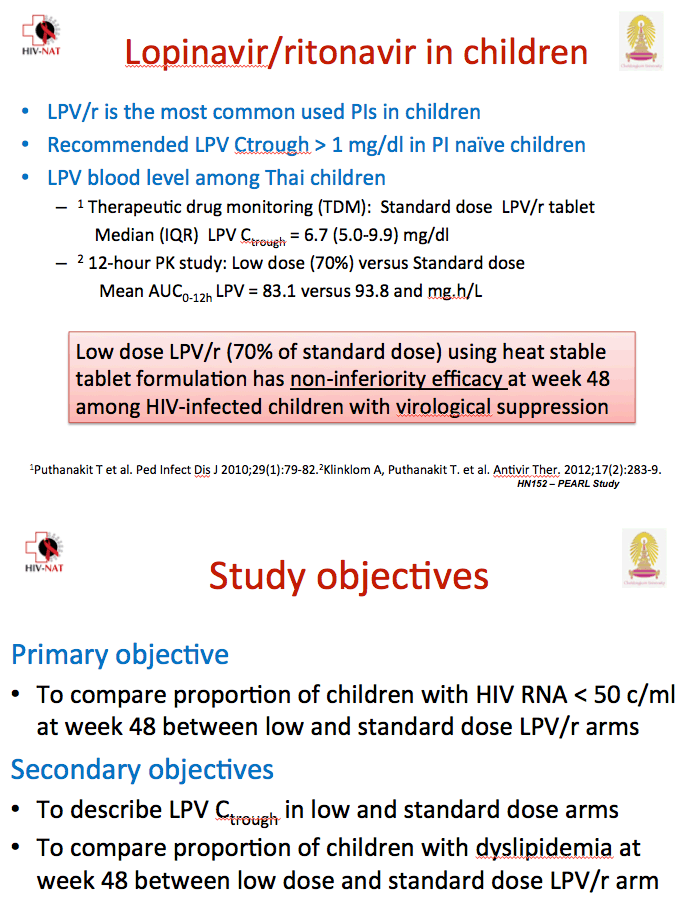
Previous research documented similar lopinavir exposure with standard-dose lopinavir/ritonavir and a 70% dose in Thai adolescents [2]. To determine whether the 70% dose maintains virologic suppression as well as continued full-dose lopinavir/ritonavir, Thai investigators conducted this multicenter, randomized, open-label trial in children with a viral load below 50 copies on a protease inhibitor regimen.
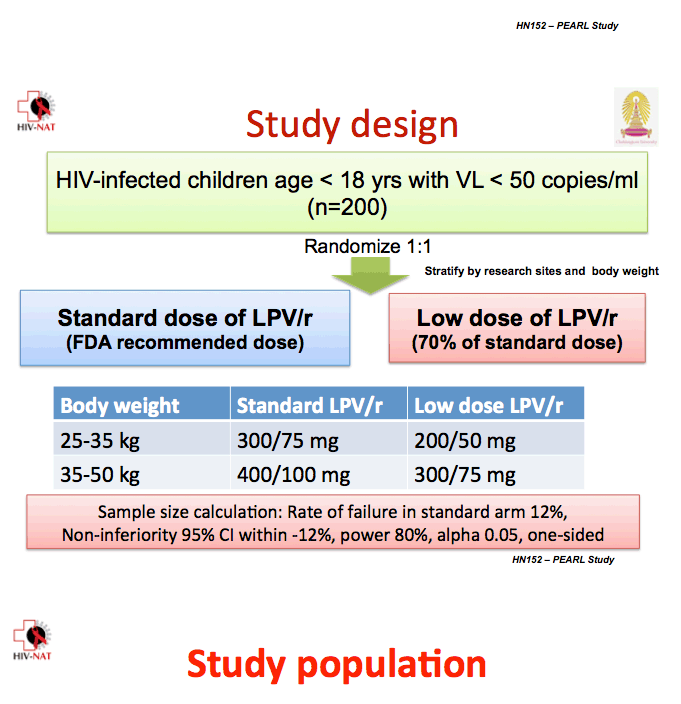
All study participants were younger than 18 and weighed between 25 and 50 kg. The study excluded children with evidence of resistance to protease inhibitors, those taking two protease inhibitors, and those taking drugs that may interact with lopinavir/ritonavir, rifampin, nevirapine, or efavirenz. The investigators randomized 199 children to standard-dose lopinavir/ritonavir or to the 70% dose.
Children weighing 25 to 35 kg took 300/75 mg or 200/50 mg twice daily, and children weighing 35 to 50 kg took 400/100 mg or 300/75 mg twice daily.
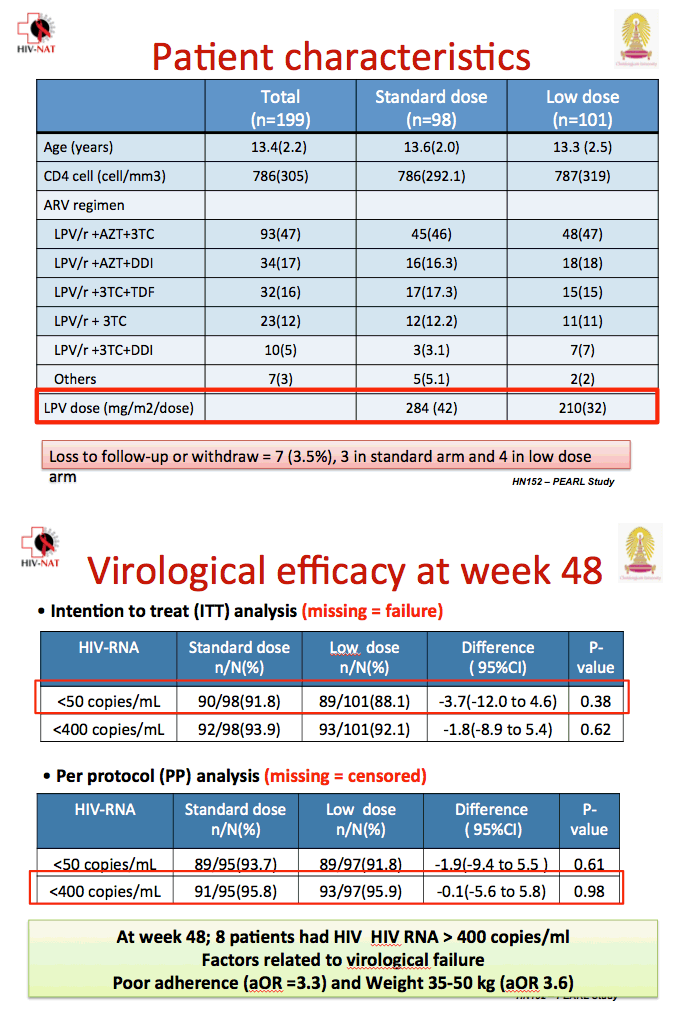
The treatment groups did not differ substantially in age (average 13.4) or CD4 count (average 786). All but 7 children (3%) were already taking lopinavir/ritonavir, usually with zidovudine/lamivudine (47%), zidovudine/didanosine (17%), or tenofovir/lamivudine (16%). Seven children dropped out of the study, 3 in the standard-dose arm and 4 in the low-dose arm.
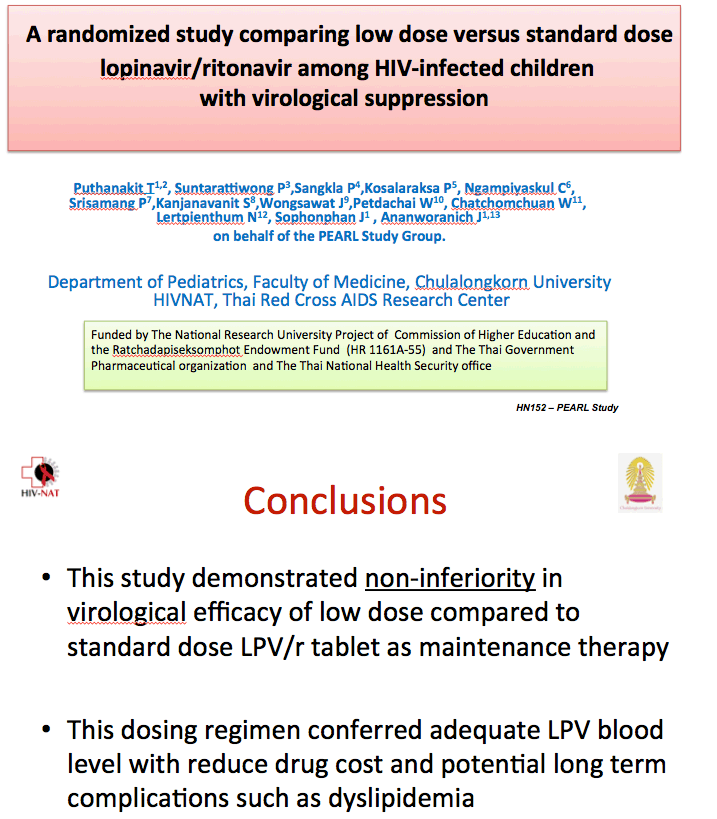
In a missing-data-equal-failure analysis, 90 of 98 children in the standard-dose group and 89 of 101 in the low-dose group had viral load below 50 copies at week 48 (91.8% versus 88.1%, difference -3.7%, 95% confidence interval [CI] -12.0 to 0.46, P = 0.38). The groups did not differ in proportions with a 48-week viral load below 400 copies. In a missing-data-equal-censored analysis, 89 of 95 in the standard-dose arm and 89 of 97 in the low-dose arm had a week-48 viral load below 50 copies (93.7% versus 91.8%, difference -1.9, 95% CI -9.4 to 5.5, P = 0.61).
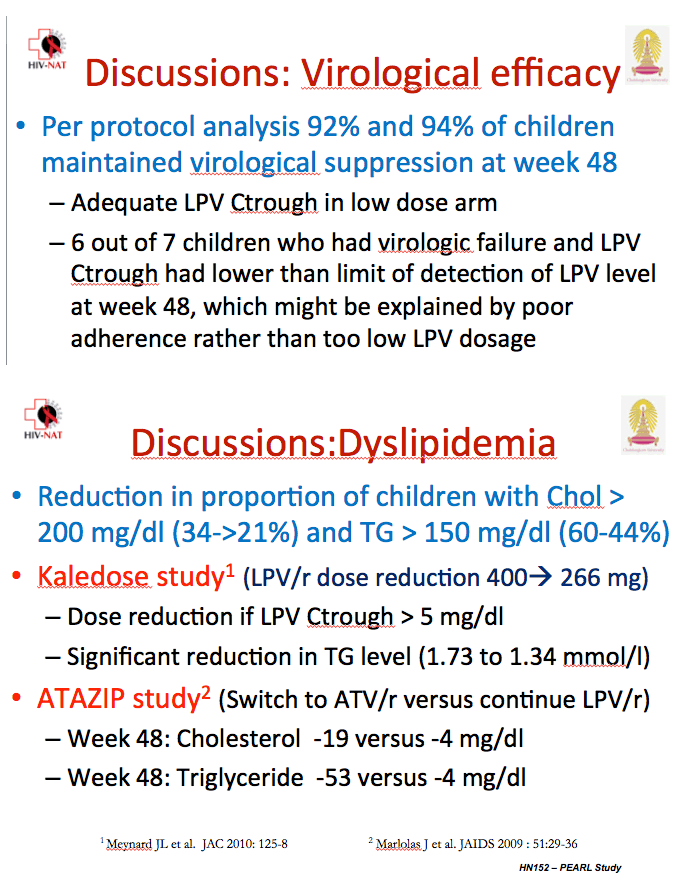
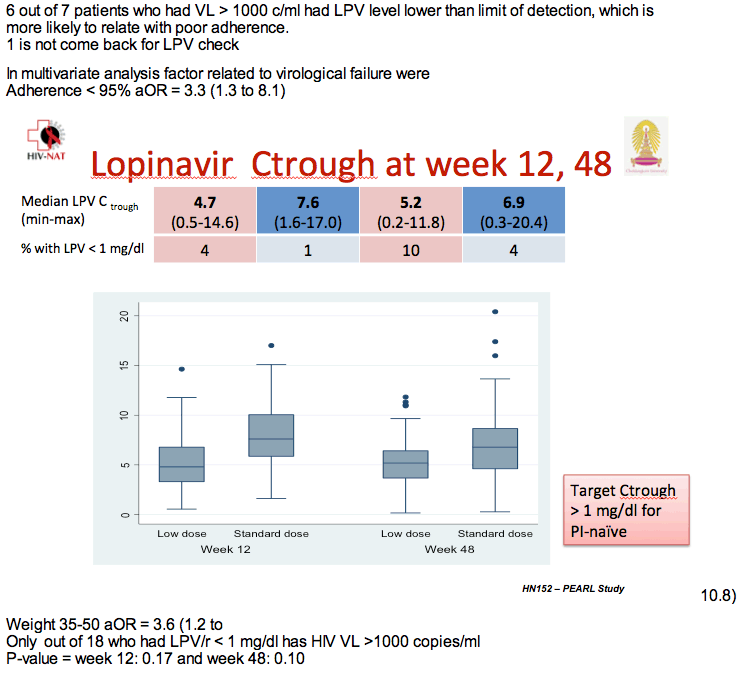
Six of 7 children with a viral load above 1000 copies at week 48 had undetectable lopinavir concentrations, a finding probably reflecting lack of adherence. At week 48 median lopinavir trough stood at 6.9 mg/dL in the standard-dose arm (range 0.3 to 20.4) and 5.2 mg/dL in the low-dose arm (range 0.2 to 11.8), a nonsignificant difference. But 4 children in the standard-dose group and 10 in the low-dose group had a lopinavir trough below 1 mg/dL at week 48.
Multivariate analysis determined that poor adherence more than tripled the odds of virologic failure (adjusted odds ratio 3.3, 95% CI 1.3 to 8.1), as did weight between 35 to 50 kg (adjusted odds ratio 3.6, 95% CI 1.2 to 10.8).
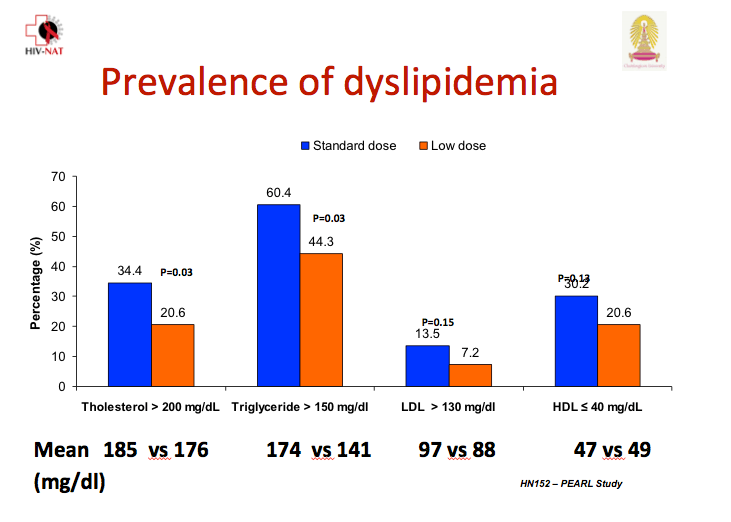
At week 48 significantly more children in the standard-dose arm had a total cholesterol above 200 mg/dL (34.4% versus 20.6%, P = 0.03), and significantly more had triglycerides above 150 mg/dL (60.4% versus 44.3%, P = 0.03). Low- and high-density lipoprotein cholesterol were nonsignificantly higher in the standard-dose group. The investigators did not report change in total-to-HDL cholesterol ratio.
The researchers concluded that a 70% dose of lopinavir/ritonavir is noninferior to the full standard dose and that the low dose yields adequate lopinavir concentrations while lowering drug costs and possibly long-term complications such as dyslipidemia.
Reference
1. Puthanakit T, Suntarattiwong P, Sangkla P, et al. A randomized study comparing low dose versus standard dose lopinavir/ritonavir among HIV-infected children with virological suppression 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur. Abstract MOAB0101.
2. Klinklom A, Puthanakit T, Gorowara M, et al. Low dose lopinavir/ritonavir tablet achieves adequate pharmacokinetic parameters in HIV-infected Thai adolescents. Antivir Ther. 2012;17:283-289.
|
| |
|
 |
 |
|
|