 |
 |
 |
| |
End-Stage Renal Disease Incidence 2 to 4 Times Higher With HIV in North America
|
| |
| |
7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur
Mark Mascolini
See Full poster report below
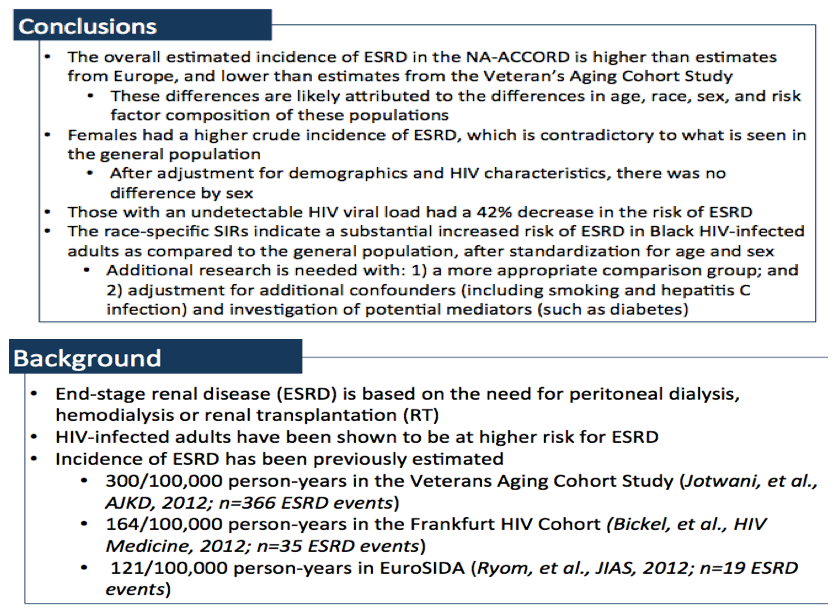
Compared with the general population, incidence of end-stage renal disease (ESRD) was twice higher in HIV-positive white members of the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) and more than 4 times higher in black members of the cohort [1]. Antiretroviral experience raised the risk of ESRD 36%, but having an undetectable viral load cut the risk 42%.
Prior HIV-related research on ESRD, defined as the need for dialysis or renal transplantation, determined an incidence (the new-event rate) ranging from 121 per 100,000 person-years in EuroSIDA, to 164 per 100,000 in the Frankfurt HIV cohort, and to 300 per 100,000 in the US Veterans Aging Cohort Study. NA-ACCORD investigators conducted the study described here to chart ESRD incidence across 12 North American cohorts and to identify risk factors.
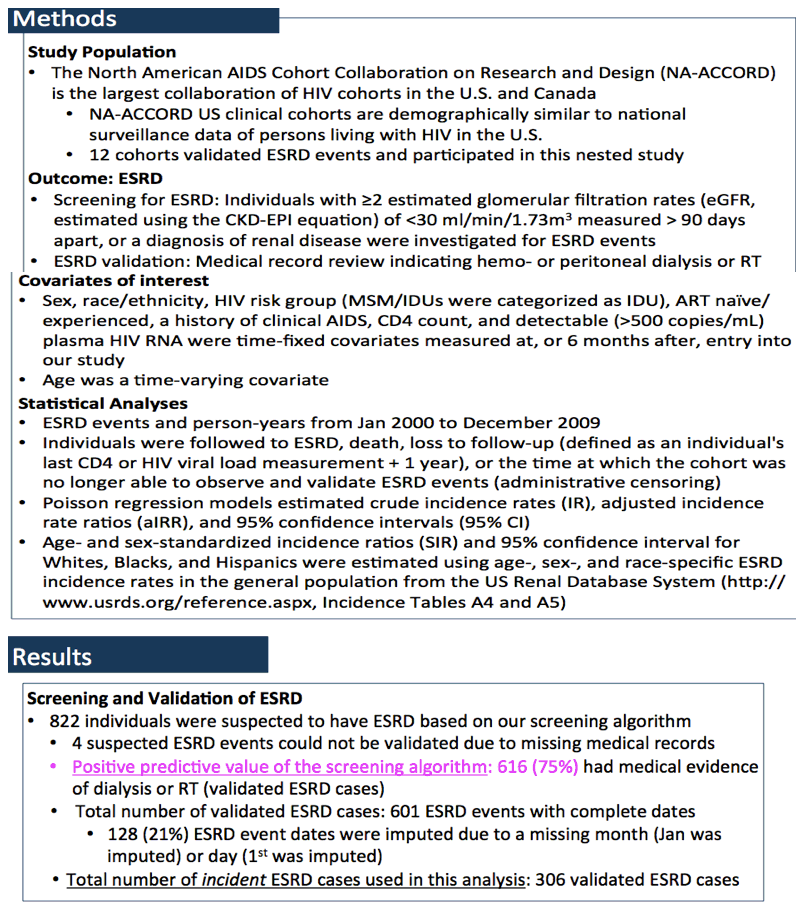
The analysis involved all HIV-positive people in NA-ACCORD. The researchers identified suspected ESRD as (1) two or more estimated glomerular filtration rates (eGFRs) below 30 mL/min and measured more than 90 days apart, or (2) a diagnosis of renal disease. They validated cases of ESRD by medical records indicating hemodialysis, peritoneal dialysis, or kidney transplant.
Statistical analysis to identify ESRD predictors included baseline or 6-month assessment of sex, race/ethnicity, HIV transmission risk group, antiretroviral experience, clinical AIDS, CD4 count, and viral load above 500 copies. The analysis considered age as a time-varying covariate. The NA-ACCORD team calculated age- and sex-standardized incidence ratios compared with rates in the general population as recorded in the US Renal Database System (http://www.usrds.org/reference.aspx, Tables A4 and A5).
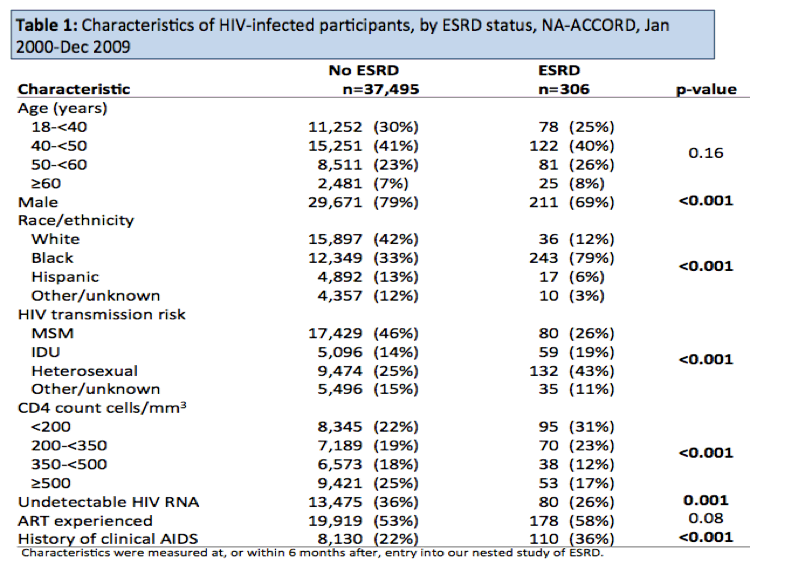
Over a study period from January 2000 to December 2009, the investigators identified 306 people with ESRD and 37,495 who remained free of ESRD. The ESRD group included a lower proportion of men than the no-ESRD group (69% versus 79%, P < 0.001), a higher proportion of blacks (79% versus 33%, P < 0.001), a lower proportion of people infected during sex between men (26% versus 46%, P < 0.001), a higher proportion infected during heterosexual sex (43% versus 25%, P < 0.001), a lower proportion with an undetectable viral load (26% versus 36%, P = 0.001), and a higher proportion with a clinical AIDS history (36% versus 22%). Lower CD4 counts were more frequent in people with ESRD. And there was a trend to a higher proportion with antiretroviral experience in the ESRD group (58% versus 53%, P = 0.08).
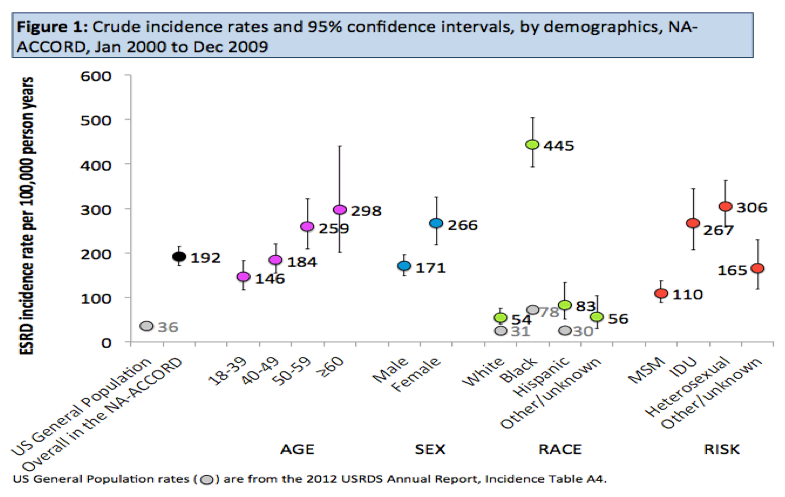
Crude ESRD incidence measured 192 per 100,000 person-years, meaning 192 of every 100,000 HIV-positive people progressed to ESRD every year. In comparison, ESRD incidence in the general US population is 36 per 100,000 person-years, one-fifth the rate in this HIV group. ESRD incidence in people with HIV rose from 146 per 100,000 person-years in 18- to 39-year-olds to 184 in 40- to 49-year-olds, 259 in 50- to 59-year-olds, and 298 in people 60 and older.
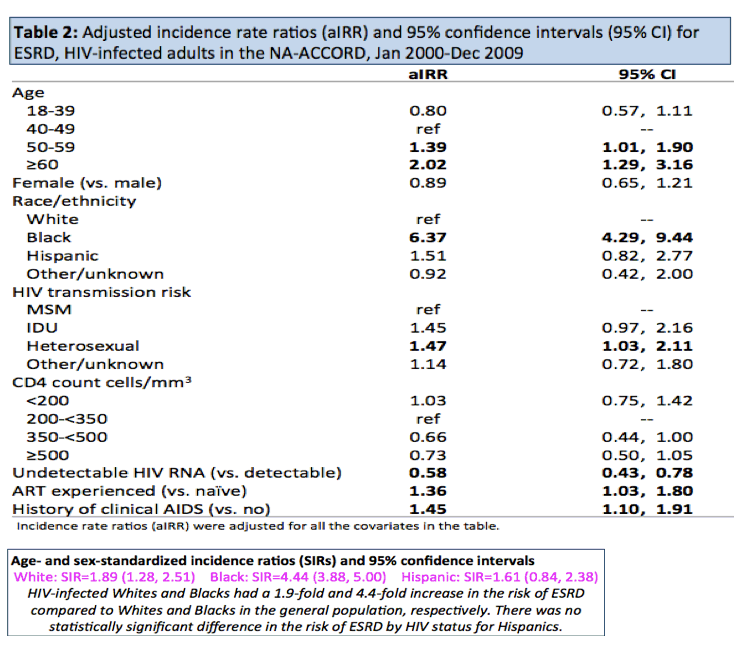
The general population has an ESRD incidence of 31 per 100,000 in whites and 78 per 100,000 in blacks. Incidence in people with HIV was 54 per 100,000 in whites and 445 per 100,000 in blacks. Age- and sex standardized incidence ratios (SIRs) indicated that HIV-positive whites in this group had almost a doubled rate of ESRD compared with whites in the general population (SIR 1.89, 95% confidence interval [CI] 1.28 to 2.51), HIV-positive Hispanics had about a 60% higher rate (SIR 1.61, 95% CI 0.84 to 2.38, not significant), and HIV-positive blacks had more than a 4 times higher rate (SIR 4.44, 95% CI 3.88 to 5.00).
Adjusted incidence rate ratios (aIRR) showed that age over 50, heterosexual HIV transmission, black race, antiretroviral experience, and a history of AIDS all independently predicted ESRD, while an undetectable viral load trimmed the risk about 40%:
-- Age 50-59 vs 40-49: aIRR 1.39, 95% CI 1.01 to 1.90
-- Age 60+ vs 40-49: aIRR 2.02, 95% CI 1.29 to 3.16
-- Black vs white: aIRR 6.37, 95% CI 4.29 to 9.44
-- Heterosexual vs homosexual HIV transmission: aIRR 1.47, 95% CI 1.03 to 2.11
-- Antiretroviral experience vs naive: aIRR 1.36, 95% CI 1.03 to 1.80
-- History of clinical AIDS: aIRR 1.45, 95% CI 1.10 to 1.91
-- Undetectable vs detectable viral load: aIRR 0.58, 95% CI 0.43 to 0.78
ESRD incidence in NA-ACCORD was higher than in the two European studies and lower than in US veterans. The researchers attributed these differences to differences in age, race, sex, and risk factors in the three study populations.
The NA-ACCORD team sees a need for additional research with (1) a more appropriate comparison group, (2) adjustment for additional variables such as smoking and hepatitis C infection, and (3) investigation of potential ESRD mediators, such as diabetes.
Reference
1. Althoff K, Abraham A, Estrella M, et al. Incidence of end stage renal disease in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur, Abstract MOPE082.
-------------------------------
Incidence of End-stage Renal Disease (ESRD) in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD)
Reported by Jules Levin
7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur
Keri N Althoff1, Alison G Abraham1, Michelle M Estrella1, Heidi M Crane2, Aimee Freeman1, M John Gill3, Elizabeth T Golub1, Michael Horberg4, Lisa P Jacobson1, Mari M Kitahata2, Rosemary McKaig5, Frank Palella6, Chirag R Parikh7, Richard D Moore1, Yuezhou Jing1, Benigno Rodriguez8, Joseph Eron9, Sonia Napravnik9, Michael J Silverberg10, Angel Mayor11, Zipporah Krishnasami12, and Greg Lucas1 for the North American AIDS Cohort Collaboration on Research and Design of IeDEA
1Johns Hopkins University, Baltimore, USA; 2. University of Washington, Seattle, USA; 3. University of Calgary, Calgary, Canada; 4. Kaiser Permanente Mid-Atlantic Research Institute, Rockville, USA; 5. National Institute of Allergy and Infectious Diseases, Bethesda, USA; 6. Northwestern University, Chicago, USA; 7. Yale University, New Haven, USA; 8. Case Western Reserve University, Cleveland, USA; 9. University of North Carolina, Chapel Hill, USA; 10. Kaiser Permanente Northern California, Oakland, USA; 11. Universidad Central del Caribe, Bayamon, USA; 12. University of Alabama, Birmingham, USA
|
| |
|
 |
 |
|
|