 |
 |
 |
| |
Quick CNS Improvement After Switch From Efavirenz to Raltegravir
|
| |
| |
7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur
Mark Mascolini
Switching from efavirenz to raltegravir led to quick improvement in central nervous system (CNS) side effects, and all 40 study participants maintained virologic suppression through 12 weeks [1].
Efavirenz is a preferred and much-used component of first-line regimens throughout the world. Short- and long-term CNS side effects are the principal drawback of efavirenz, and differentiating these problems from background psychiatric problems can be difficult. Raltegravir has also proved a potent first-line antiretroviral and has a better safety record than efavirenz. Clinical researchers at London's Chelsea and Westminster Hospital conducted this open-label study to assess changes in CNS toxicity and virologic response in people switching from efavirenz to raltegravir while maintaining the other antiretrovirals in their regimen.
The study involved 40 people with virologic suppression and persistent efavirenz-related CNS toxicity. Everyone swapped efavirenz for raltegravir and kept taking their nucleosides for 12 weeks. The primary endpoint was a CNS toxicity score 4 weeks after the switch calculated by the ACTG adverse event score and the Sleep Questionnaire. The CNS score includes 10 items related to potential efavirenz side effects: dizziness, depression, insomnia, anxiety, confusion, impaired concentration, headache, somnolence, aggressive mood, and abnormal dreams. Each side effect is scored 0 for none, 1 for mild, 2 for moderate, and 3 for severe. The Sleep Questionnaire includes 19 items; a lower score indicates better sleep. The researchers converted absolute CNS toxicity and sleep score to a percentage.
Thirty-eight of the 40 study participants were men, and 34 were white. Age averaged 43 years (range 29 to 62), and median time taking efavirenz stood at 27.5 months (range 4 to 145). Median CD4 count at the switch measured 550 (interquartile range [IQR] 423 to 720).
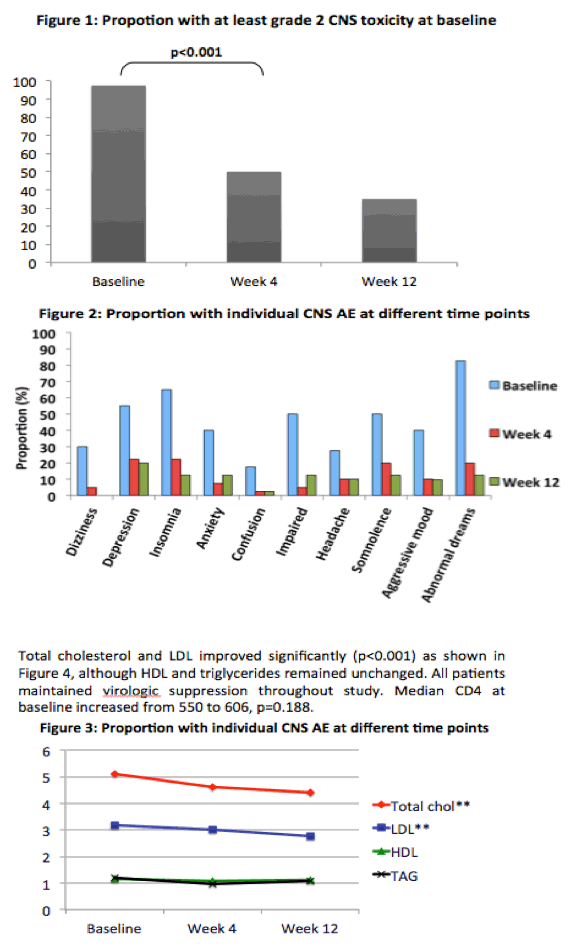
Median total CNS score dropped from 45 (IQR 30 to 60) at the switch to 19 (IQR 9 to 29) at week 4 (P < 0.001) and to 13 (IQR 3 to 23) at week 12 (P < 0.001). All components in the CNS score except headache improved significantly. Median total Sleep Questionnaire score improved from 34.7 (IQR 28.6 to 40) at the switch to raltegravir to 21.9 (IQR 17.2 to 28.2) at week 4 and to 20.4 (IQR 12.6 to 28.6) at week 12 (P < 0.001).
Total cholesterol and "bad" low-density lipoprotein cholesterol improved significantly through 12 weeks on raltegravir. "Good" high-density lipoprotein cholesterol and triglycerides did not change significantly in that time. All study participants maintained virologic suppression through 12 weeks of follow-up, and median CD4 count rose from 550 at the switch to 606 at week 12, a nonsignificant change (P = 0.188).
The CNS improvements recorded in this study reflect results of a previous double-blind trial of a switch from efavirenz to another nonnucleoside, etravirine [2]. In the efavirenz-etravirine switch study, only insomnia, abnormal dreams, and nervousness improved significantly after the switch.
The Chelsea and Westminster investigators suggested that "identification of individuals with efavirenz toxicity is essential as switching to alternative agents may lead to improvements in toxicity profile [and] quality of life" and so affect long-term adherence."
References
1. Yapa HM, Waters L, Rowlands J, et al. The feasibility of switching efavirenz (EFV)-based highly active antiretroviral therapy to raltegravir (RAL)-based therapy in HIV-infected individuals with central nervous system (CNS) toxicity: a phase IV open label pilot study. 7th IAS Conference on HIV Pathogenesis, Treatment and Prevention, June 30-July 3, 2013, Kuala Lumpur. Abstract MOPE090.
2. Water L, Fisher M, Winston A, et al. A phase IV, double-blind, multicentre, randomized, placebo-controlled, pilot study to assess the feasibility of switching individuals receiving efavirenz with continuing central nervous system adverse events to etravirine. AIDS. 2011;25:65-71.
of switching individuals receiving efavirenz with continuing central nervous system adverse events to etravirine. AIDS. 2011;25:65-71
-------------------------
A phase IV open label single centre pilot switch study was performed in individuals with virologic suppression and persistent CNS toxicity potentially due to EFV
primary endpoint was the rate of CNS toxicity at week 4 calculated by the ACTG adverse event (AE) score and Sleep Questionnaire (SQ).
Secondary endpoints were: rate of CNS toxicity at week 12, continued virologic suppression, change in CD4 count and fasting lipids week 4 and week 12 compared with baseline
AUTHOR CONCLUSIONS
This study demonstrated significant improvements in CNS toxicities and sleep quality over a 12 week period in individuals switching from EFV to RAL. As an open label study, there is potential for bias. However the findings are consistent with a previous double-blinded switch study from EFV to etravirine [1] although for individual AE that study only insomnia, abnormal dreams and nervousness improved significantly. The more widespread improvement in CNS toxicity parameters observed in the current study, may be attributable to a change in drug class.
Switching EFV to RAL led to significant improvement in CNS toxicity and sleep quality, with maintainance of virologic suppression. Identification of individuals with EFV toxicity is essential as switching to alternative agents may lead to improvements in toxicity profile, quality of life and impact on long term adherence.
|
| |
|
 |
 |
|
|