 |
 |
 |
| |
CLINICAL EFFICACY OF HIGHLY EFFECTIVE INTERFERON-FREE THERAPY IN PATIENTS WITH CHRONIC HCV INFECTION AND COMPENSATED ADVANCED HEPATIC FIBROSIS - Life Expectancy in Patients With Chronic HCV Infection and Cirrhosis Compared With a General Population
|
| |
| |
Download the PDF here
---------------------
AND in JAMA
Life Expectancy in Patients With Chronic HCV Infection and Cirrhosis Compared With a General Population (same HCV+ patient cohort as presented in this oral slide presentation at AASLD)
Research Letter | November 12, 2014
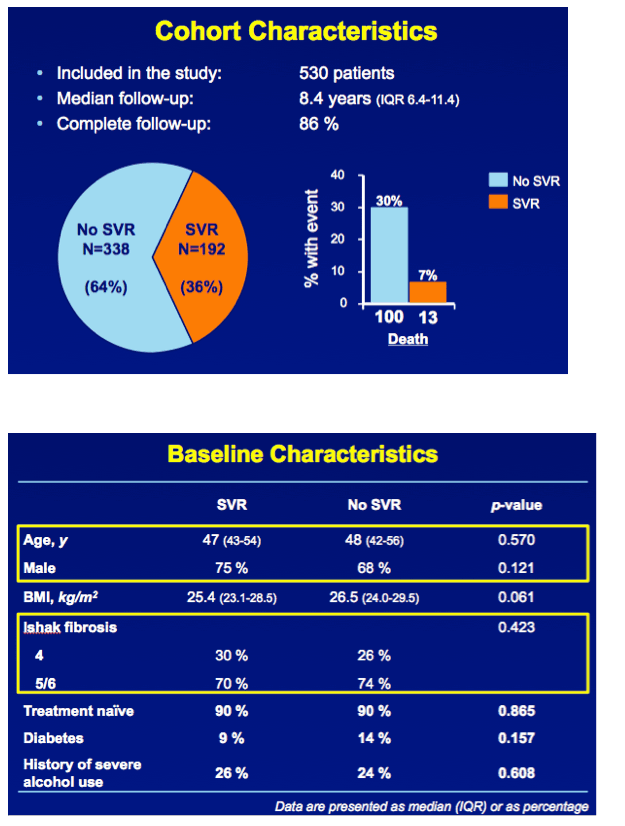
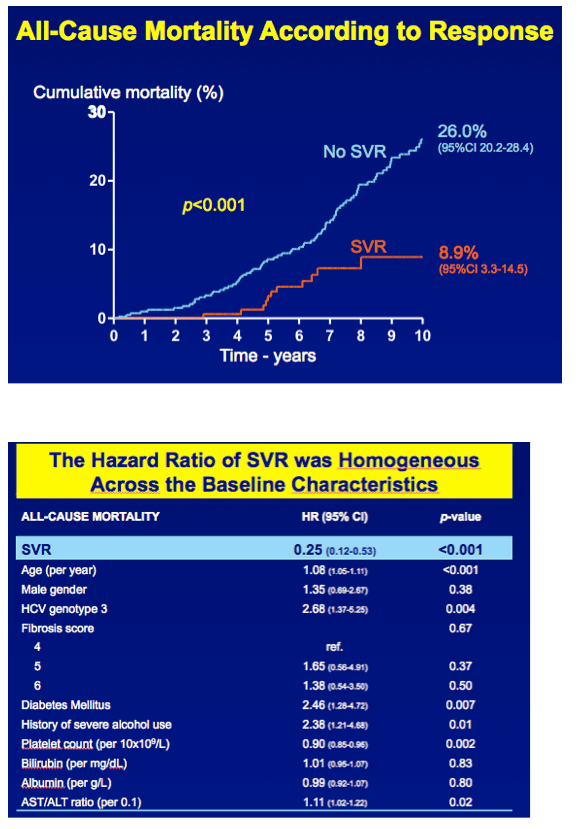
"In total, 530 patients were followed up for a median of 8.4 years (interquartile range [IQR], 6.4-11.4 years). Follow-up was complete in 454 patients (86%). The median age was 48 years (IQR, 42-56 years), 369 patients (70%) were male, and 192 attained SVR (36%). Thirteen patients with SVR died, resulting in a cumulative 10-year overall survival of 91.1% (95% CI, 85.5%-96.7%), which did not differ significantly from the age- and sex-matched general population (P = .57) (Figure)."
Adriaan J. van der Meer, MD, PhD1; Heiner Wedemeyer, MD, PhD2; Jordan J. Feld, MD3; Jean-François Dufour, MD, PhD4; Stefan Zeuzem, MD,
PhD5; Bettina E. Hansen, PhD1; Harry L. A. Janssen, MD, PhD1
See the full text of this article below following the slide presentation
----------------------------------
Reported by Jules Levin
AASLD 2014 Nov 7-11 Boston
A.J. van der Meer1, R. Maan1, J.J. Feld2, H. Wedemeyer3, G. Fattovich4,
J.-F. Dufour5, F. Lammert6, A. Duarte-Rojo2, M.P. Manns3, S. Zeuzem7, W.P. Hofmann7, D. Ieluzzi4, R.J. de Knegt1, B.E. Hansen1, B.J. Veldt1, and
H.L.A. Janssen1,2
Program Abstract
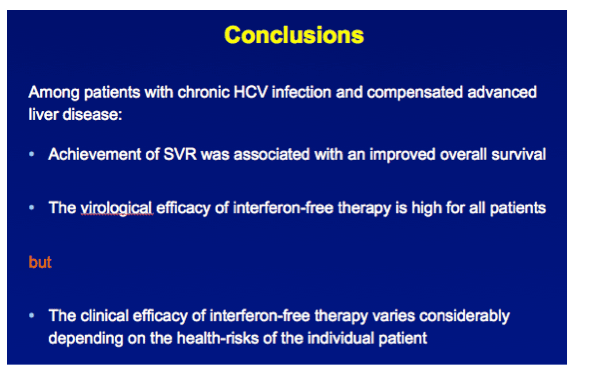
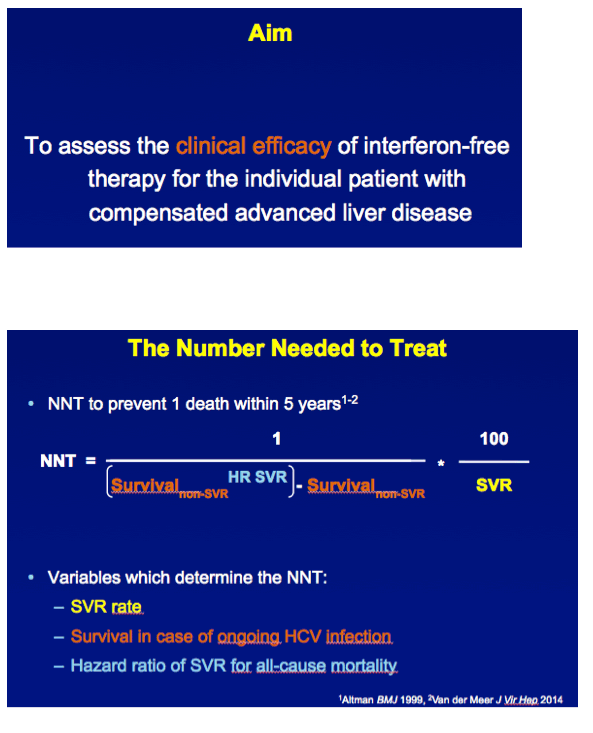
INTRODUCTION Independent of host characteristics, 95% of patients with chronic HCV infection attain SVR with interferon-free therapy. We aimed to assess the clinical efficacy of such therapies for the individual patient with compensated advanced fibrosis.
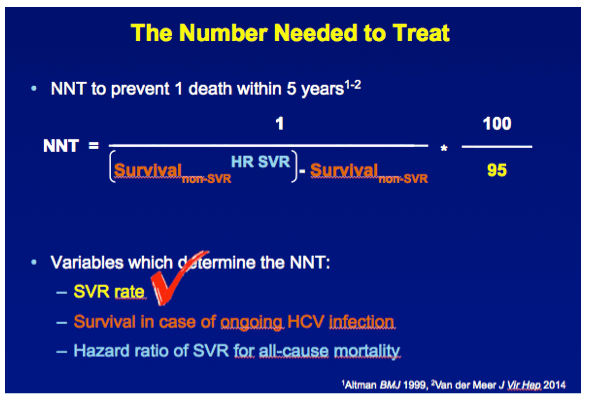
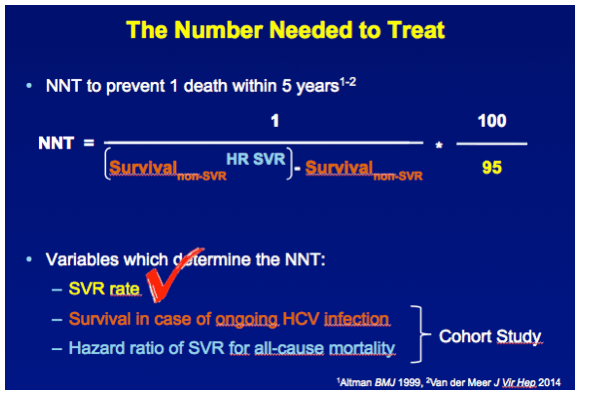
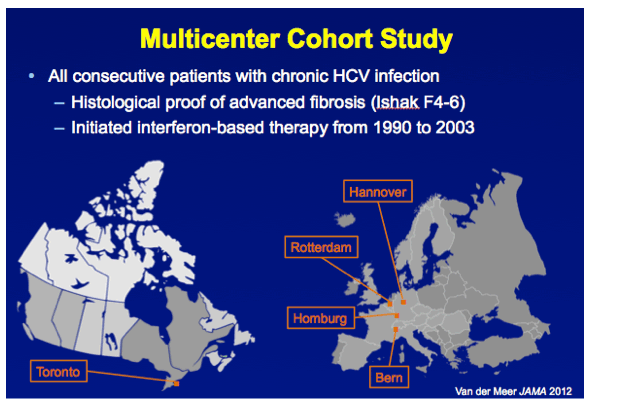
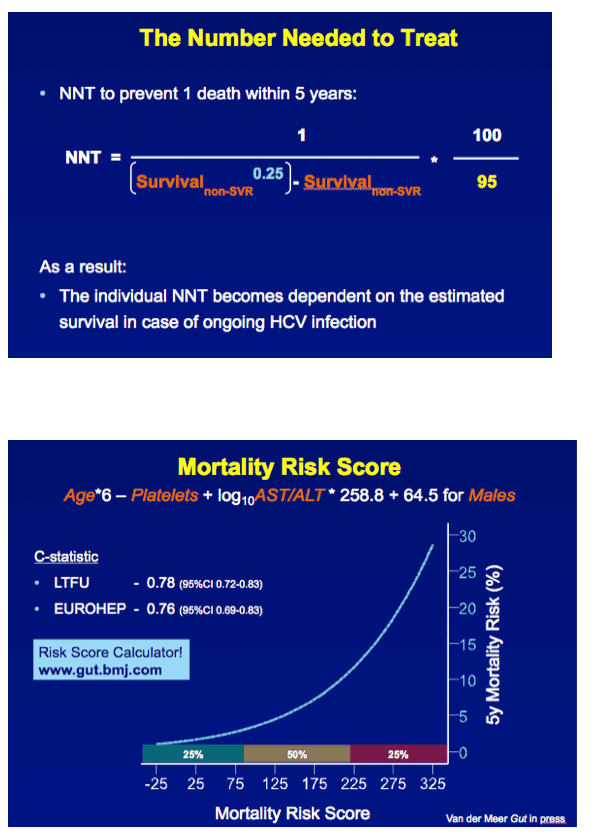
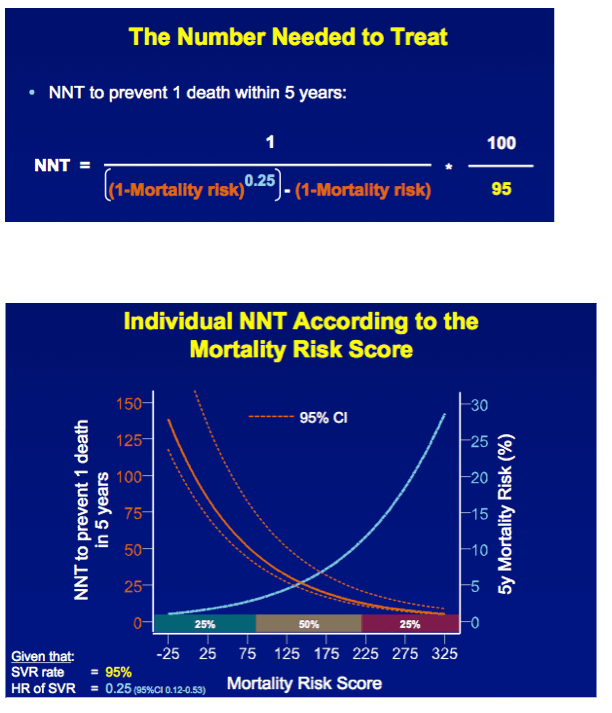
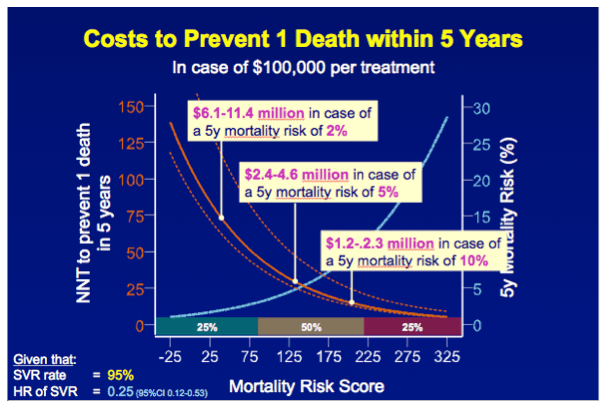
METHODS A multicenter cohort study including all consecutively treated patients with chronic HCV infection and advanced hepatic fibrosis was performed. Clinical efficacy of therapy was assessed as the number needed to treat (NNT) to prevent 1 death in 5 years, which was calculated with the adjusted hazard ratio (HR) of SVR for all-cause mortality and the individual's estimated 5-year survival based on our externally validated mortality risk score (including solely objective variables). [NNT=(1/(estimated 5y-survival without SVR^(HR of SVR) - estimated 5y-survival without SVR))*(100/SVR rate)]
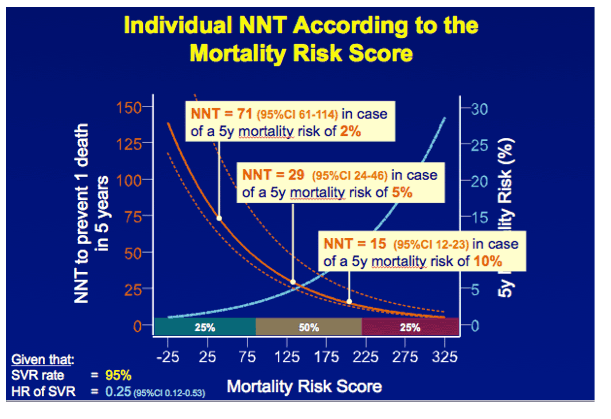
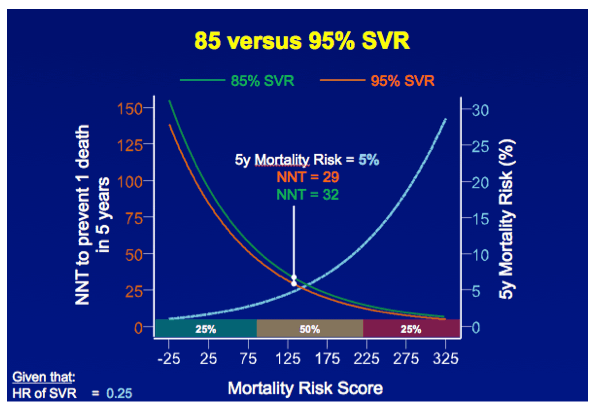
RESULTS In total, 530 patients were followed for a median of 8.4 (IQR 6.4-11.4) years. Median age was 48 (IQR 42-56) years, 143 (27%) patients had bridging fibrosis and 387 (63%) had cirrhosis. SVR was attained by 192 (36%) patients. Cox analyses showed that SVR was independently associated with reduced all-cause mortality (adjusted HR 0.25, 95%CI 0.12-0.53), without significant interactions with any baseline variables. Among patients without SVR, the 5-year mortality rate was 8.6 (95%CI 5.7-11.5). For calculating the NNT, the HR of SVR was fixed at 0.25 and the SVR rate at 95%. The NNT to prevent 1 death in 5 years was 29, 15, 10 or 8 in case of a 5-year mortality risk of 5, 10, 15 or 20%, respectively. The figure shows the estimated NNT and 5-year mortality risk, according to the individual's mortality risk score.
CONCLUSION These results indicate that the clinical efficacy of interferon-free therapy varies extensively among patients with advanced liver disease, which might provide guidance when prioritizing patients for treatment with these costly regimens.
-----------------------------
JAMA. November 12, 2014
Life Expectancy in Patients With Chronic HCV Infection and Cirrhosis Compared With a General Population
Adriaan J. van der Meer, MD, PhD1; Heiner Wedemeyer, MD, PhD2; Jordan J. Feld, MD3; Jean-François Dufour, MD, PhD4; Stefan Zeuzem, MD, PhD5; Bettina E. Hansen, PhD1; Harry L. A. Janssen, MD, PhD1
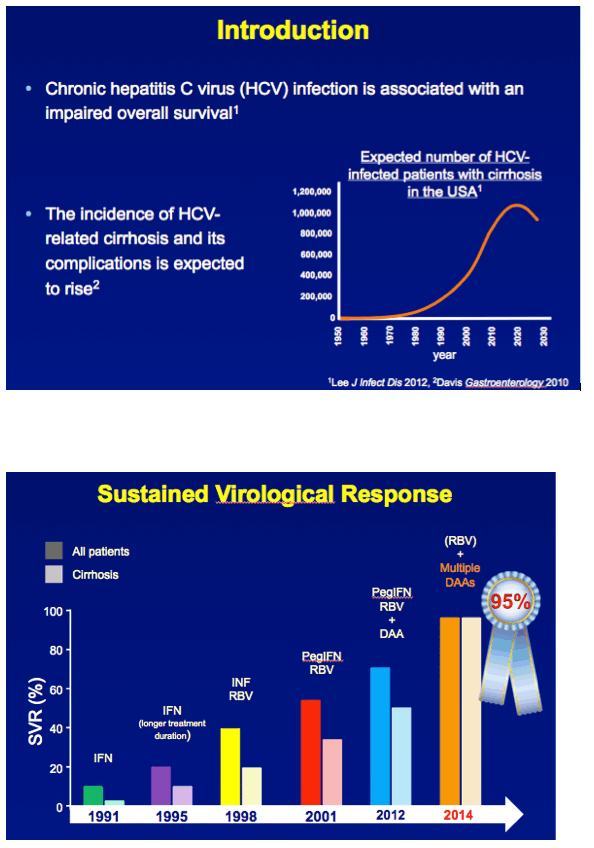
Almost 3 million people in the United States are chronically infected with the hepatitis C virus (HCV).1 The life expectancy of patients with chronic HCV infection is reduced compared with the general population, largely attributable to the development of cirrhosis, liver failure, and hepatocellular carcinoma.2
We3 and others have shown that the hazard of all-cause mortality is lower among patients with chronic HCV infection and advanced hepatic fibrosis if sustained virological response (SVR) is attained, but comparisons have been limited to those without SVR.3 To further investigate the advantage of attaining SVR, we compared overall survival of patients with chronic HCV infection and bridging fibrosis or cirrhosis before therapy (with and without SVR) with that of the general population.
METHODS
We used data on patients with chronic HCV and advanced hepatic fibrosis from a previous study; the design and characteristics of the population have been described in detail.3 The ethics committees at the individual centers approved the study. Written or oral informed consent was obtained from all patients.
All consecutive patients with chronic HCV monoinfection and biopsy-proven advanced hepatic fibrosis (Ishak fibrosis scores 4, 5, or 6) who initiated interferon-based antiviral therapy between 1990 and 2003 from 5 large hepatology units in Europe and Canada were included. Follow-up started 24 weeks after cessation of antiviral treatment, at which time achievement of SVR (defined as HCV RNA negativity in a blood sample using molecular assays) was determined. If the survival status as of January 1, 2010, could not be determined from retrospective chart review, the patient, primary care physician, or both were recontacted. If this was not successful by October 2011, the follow-up was considered incomplete.
For each virological response group, the observed overall survival was compared with the expected survival from matched age-, sex- and calendar time-specific death rates of the general population in the Netherlands, which were obtained from the bureau of statistics, using the life table method and the Wilcoxon (Gehan) test. The statistical tests were 2-sided and P < .05 was considered statistically significant. SPSS version 21.0 (SPSS Inc) was used for the statistical analyses.
RESULTS
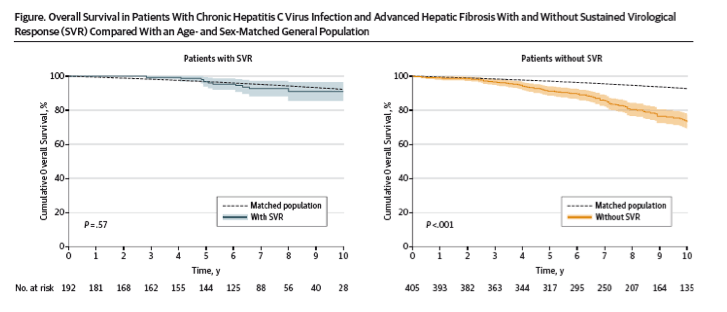
In total, 530 patients were followed up for a median of 8.4 years (interquartile range [IQR], 6.4-11.4 years). Follow-up was complete in 454 patients (86%). The median age was 48 years (IQR, 42-56 years), 369 patients (70%) were male, and 192 attained SVR (36%). Thirteen patients with SVR died, resulting in a cumulative 10-year overall survival of 91.1% (95% CI, 85.5%-96.7%), which did not differ significantly from the age- and sex-matched general population (P = .57) (Figure).
In contrast, 100 patients without SVR died. The cumulative 10-year survival was 74.0% (95% CI, 71.6%-79.8%), which was significantly lower compared with the matched general population (P < .001)
DISCUSSION
Among patients with chronic HCV infection and bridging fibrosis or cirrhosis, attaining SVR was associated with survival comparable with that of the general population, whereas not attaining SVR was associated with reduced survival. The excellent survival among patients with advanced liver disease and SVR might be explained by the associations between SVR and regression of hepatic inflammation and fibrosis, reduced hepatic venous pressure gradient, reduced occurrence of hepatocellular carcinoma and liver failure, as well as reduced occurrence of diabetes mellitus, end-stage renal disease, and cardiovascular events.4,5 Even though patients with cirrhosis and SVR remain at risk for hepatocellular carcinoma, the annual hepatocellular carcinoma incidence is low and survival is substantially better compared with those without SVR.6 Competing risks could also contribute.
Our study is limited by its retrospective design and restriction to general population data that only were available for the Netherlands. However, life expectancy in the Netherlands is similar to other participating countries so this is not expected to have had a major influence on our results. Also, all patients received interferon-based therapy. Thus, these results need to be confirmed when interferon-free therapy is widely used because these highly effective regimens may be administered to patients with more comorbidity and more advanced liver disease.
|
| |
|
 |
 |
|
|