 |
 |
 |
| |
Telaprevir in Treatment-Experienced HIV-HCV G1 Coinfected Patients (ANRS HC26 TelapreVIH)
|
| |
| |
Reported by Jules Levin
CROI 2014 March 3-6 Boston, MA
Laurent Cotte, Corine Vincent, Philippe Sogni, Isabelle Fournier, Hugues Aumaitre, Pierre De Truchis, Isabelle Rosa, Stephane Chevaliez, Jean-Pierre Aboulker, Jean-Michel Molina - ANRS HC26 study group
Program Abstract-
Background: Retreatment with PegInterferon (PegIFN) + Ribavirin (RBV) results in poor SVR rates in HIV-HCV coinfected patients. There is no data
regarding the use of Telaprevir (TVR) + PegIFN-RBV in this population
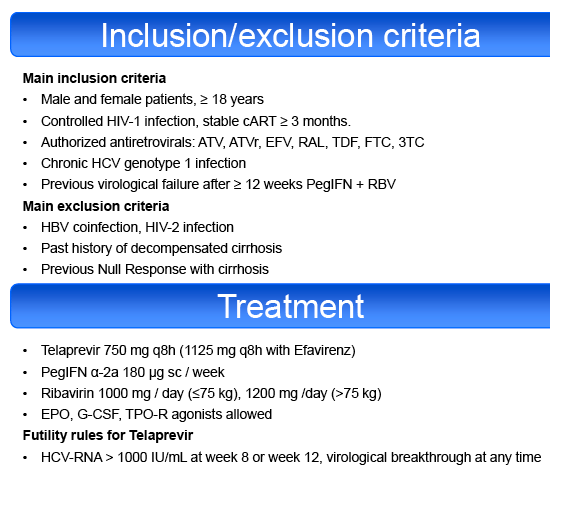
Methodology: HIV-1 infected patients who had previously failed ≥12 weeks PegIFN-RBV for HCV genotype 1 coinfection were enrolled in a single-arm,
phase 2 trial. Authorized antiretrovirals (ART) were tenofovir (TDF), emtricitabine (FTC), efavirenz (EFV), boosted atazanavir (ATVr) and raltegravir (RAL).
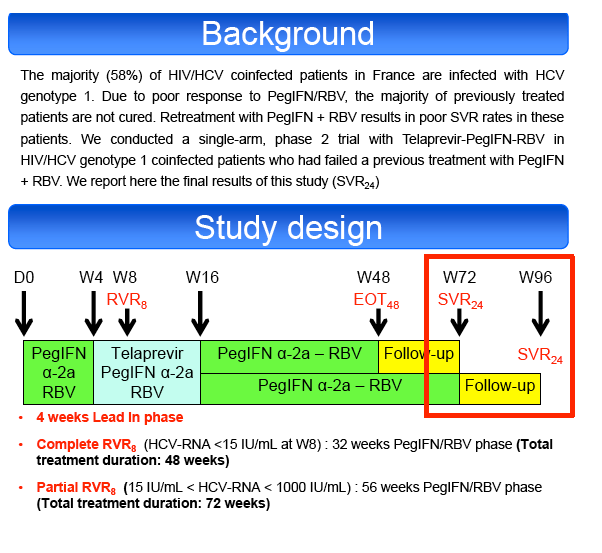
Null-responders with cirrhosis were excluded. All patients received PegIFN α2a (180μg/week) + RBV (1000-1200mg/day) for 4 weeks (lead-in phase
(LI)), followed by TVR (750 mg or 1125mg tid if EFV-based ART) + PegIFN-RBV for 12 weeks and finally PegIFN-RBV for an additional 32 to 56 weeks
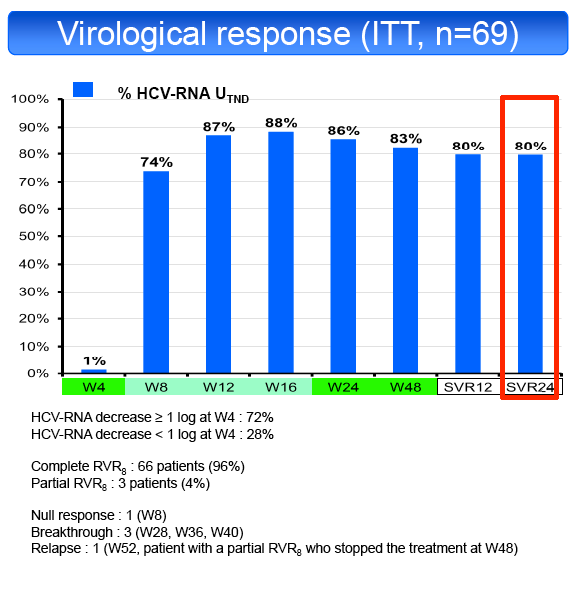
according to their virological response at week 8 (RVR8). Primary endpoint was the sustained virological response at 24 weeks post-treatment (SVR24) by
ITT analysis.
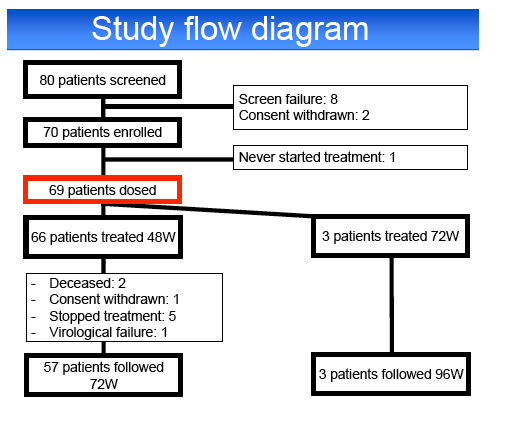
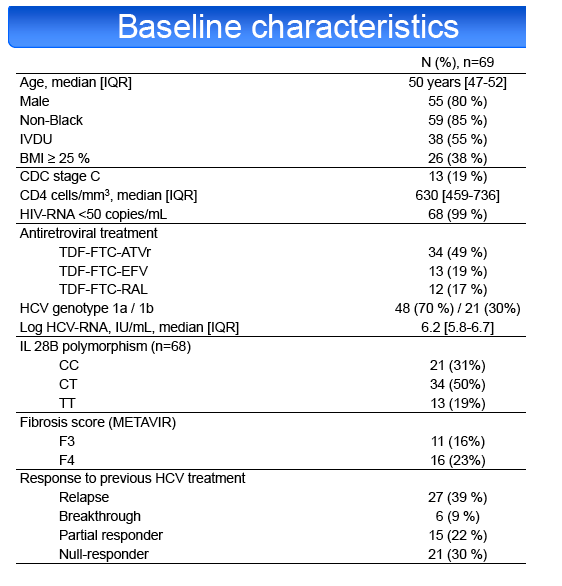
Results: Among 70 screened patients, 69 (39% relapsers (RR), 9% breakthroughs (VB), 22% partial (PR) and 30% null responders (NR)) started
treatment. Median [IQR] CD4 cell count was 630 cells/mm3 [459-736]. ART regimen was TDF-FTC with ATVr in 49%, EFV in 19%, RAL in 17% and other
combinations in 14%. HCV genotype was 1a in 70% of cases. METAVIR fibrosis stage was F3 in 16% and F4 in 23%. IL28B genotype was CC in 31%, CT
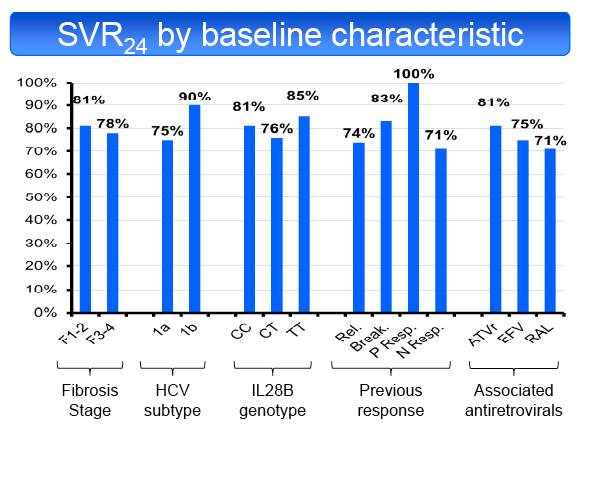
in 50% and TT in 19%. SVR24 was achieved in 55/69 patients (79.7%, 95% CI: 68.3%-88.4%). SVR24 was not influenced by fibrosis stage (F1-2 81%,
F3-4 78%), ART regimen (ATVr 81%, EFV 75%, RAL 71%), HCV subtype (1a 75%, 1b 90%), baseline CD4 cell count (<350/mm3 60%, ≥350/mm3
83%), type of previous response (RR 74%, VB 83%, PR 100%, NR 71%), baseline HCV-RNA (<5.9 Log UI/mL 71%, ≥5.9 Log UI/ml 83%), HCV-RNA
decline at the end of LI (<1 log UI/mL 74%, ≥1 log UI/ml 82%) or IL28B genotype (CC 81%, CT 76%, TT 85%). Only 3 patients with a partial RVR8 were
assigned to 72 weeks of treatment, the remaining patients were assigned to a 48 weeks treatment.
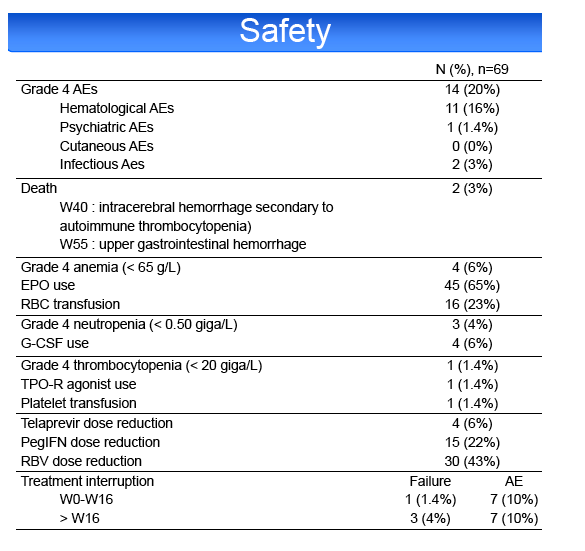
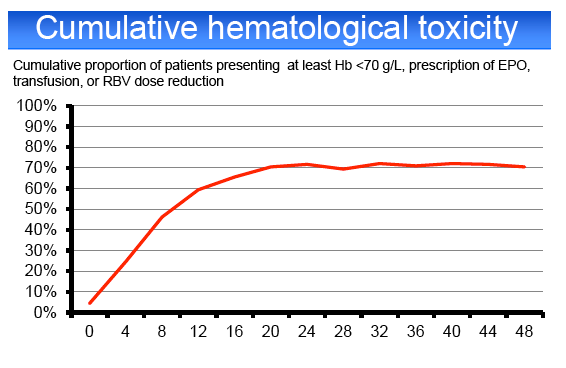
HCV treatment was prematurely discontinued for toxicity in 20% of patients, including cutaneous adverse events (AE) 4%, psychiatric AE 4%,
hematological AE 6%, other AE 6%. Grade 4 AEs occurred in 20% of cases, including anemia (10%), leuconeutropenia (6%), thrombocytopenia (1%) and
infections (3%). PegIFN or RBV dose reduction was required in 23 and 43%, respectively. 65% of the patients required EPO, 23% blood transfusions, 6%
G-CSF and 1% platelet transfusions and TPO-R agonist. Two patients died during the study, 1 from an intracerebral hemorrhage at week 40 and 1 from an
upper gastrointestinal hemorrhage at week 55.
Conclusions: Despite a high discontinuation rate related to toxicity, a high proportion of treatment-experienced HIV-coinfected patients achieved SVR24
with a TVR-based regimen.
|
| |
|
 |
 |
|
|