 |
 |
 |
| |
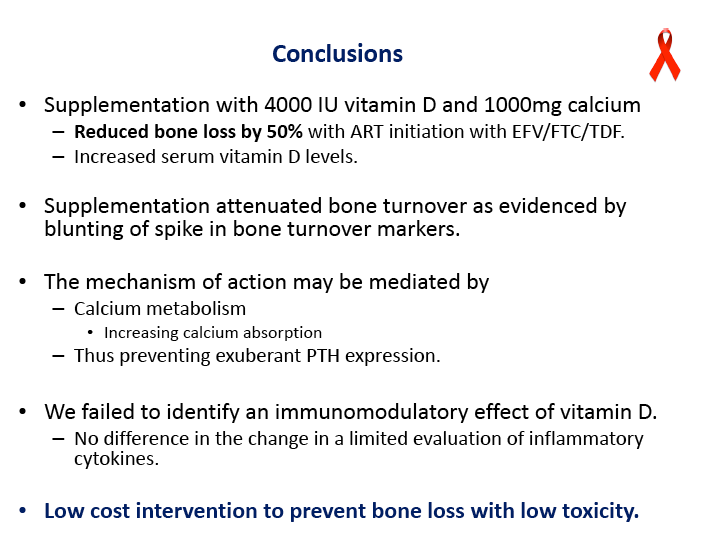
Vitamin D/Calcium Supplements Reduce Bone Loss by 50% When Starting EFV/TDF/FTC
|
| |
| |
CROI 2014, March 3-6, 2014, Boston
Mark Mascolini
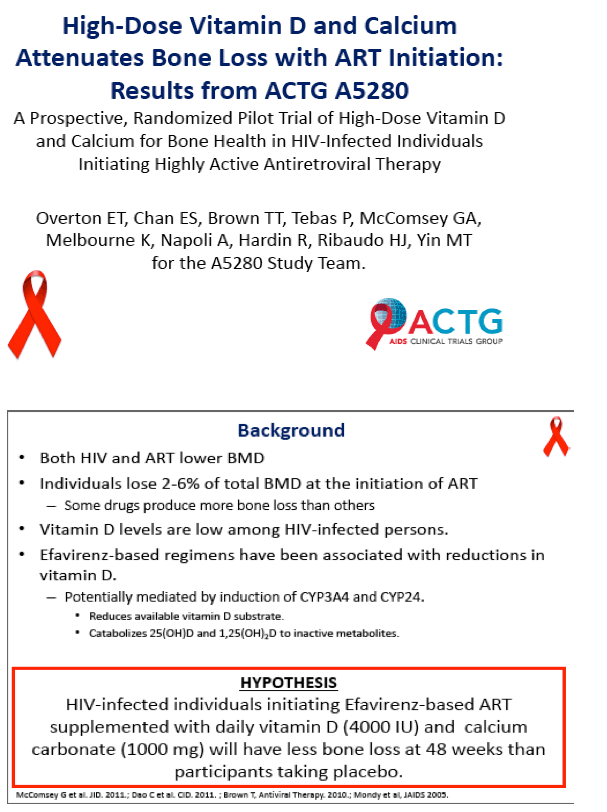
Daily high-dose vitamin D and calcium supplements reduced total hip bone loss 50% in a 48-week placebo-controlled trial that enrolled people starting efavirenz plus tenofovir/emtricitabine [1]. The 165-person ACTG A5280 trial left open questions about the vitamin D dose used and whether calcium is an essential part of the regimen.
A 2% to 6% drop in bone mineral density (BMD) when people start antiretroviral therapy is a well-documented phenomenon and is especially concerning in people with other bone risk factors and those starting tenofovir. Efavirenz has been linked to lower vitamin D, essential for healthy bones. Plentiful research documents low vitamin D levels in people with HIV and in the general population.
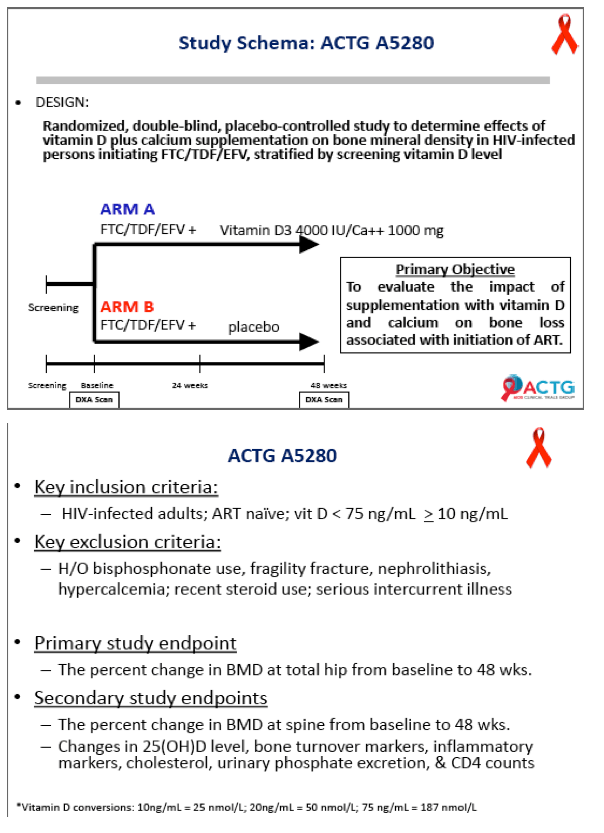
ACTG investigators conducted this double-blind placebo-controlled trial to determine whether 48 weeks of 4000 IU of vitamin D plus 1000 mg of calcium carbonate daily will ameliorate BMD loss in people starting efavirenz with tenofovir/emtricitabine. Participants had a DXA scan before starting antiretrovirals and again at 48 weeks. Participants had to be antiretroviral naive and had to have a vitamin D level between 10 and 75 ng/mL. They could not be taking bisphosphonates or recent steroids or have a history of fragility fracture, kidney stones, or hypercalcemia.
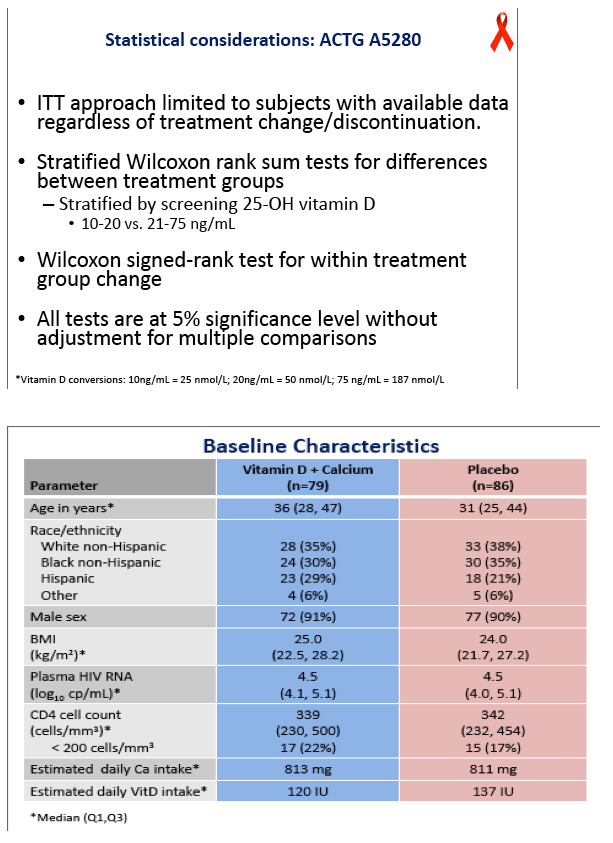
The 79 people randomized to vitamin D/calcium and the 86 randomized to placebo were similar in median age (36 and 31), race (35% and 38% white, 30% and 35% black), gender (91% and 90% men), body mass index (25 and 24 kg/m2), viral load (4.5 log10 copies/mL in each group), and CD4 count (339 and 342). Three-day diet-recall analysis indicated adequate calcium intake (813 mg daily) but low vitamin D intake (131 IU daily) in both arms.
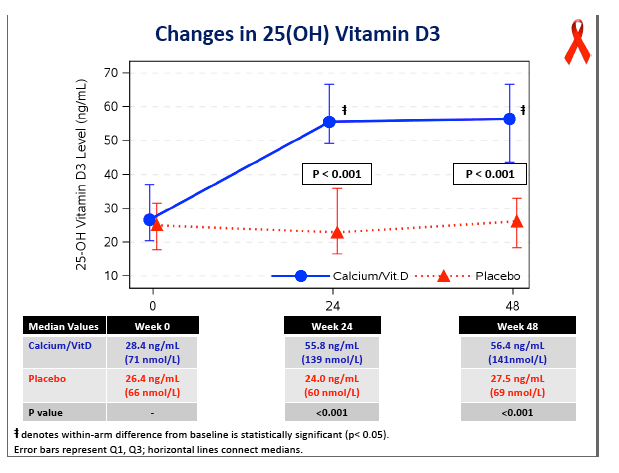
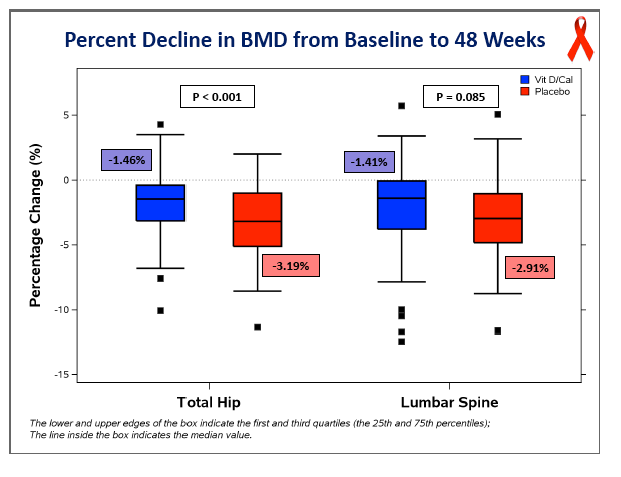
Concentrations of 25-OH vitamin D rose significantly in the D/calcium arm, from 28.4 ng/mL at baseline to 56.4 ng/mL at week 48 (P < 0.05), while levels did not change in the placebo group (P < 0.001 between groups). At week 48 total hip BMD had dropped 3.19% in the placebo group versus 1.46% in the D/calcium group, a significant difference (P < 0.001). Lumbar spine declines were 2.91% with placebo and 1.41% with D/calcium, a difference that stopped short of statistical significance (P = 0.085).
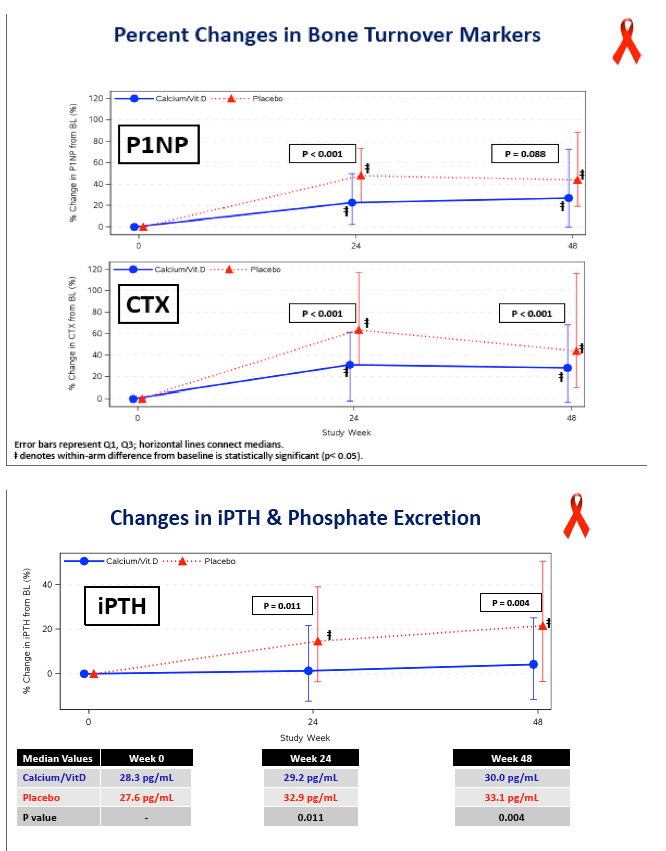
P1NP, a bone formation marker, rose significantly in both arms and nonsignificantly more with placebo (P = 0.088). CTX, a bone resorption marker, also rose significantly in both arms, and significantly more with placebo than with D/calcium (P < 0.001). This blunting of bone turnover markers with vitamin D and calcium indicates a drop in bone turnover with the intervention.
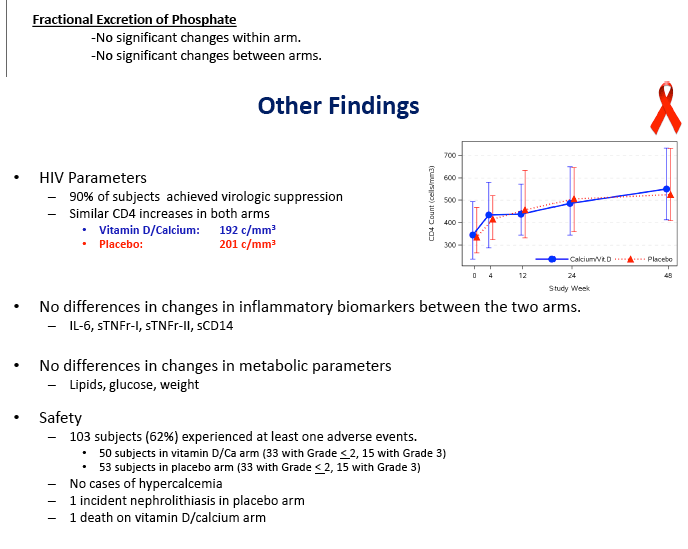
Virologic response did not differ between the two groups, with 90% overall reaching an undetectable viral load in 48 weeks. CD4 counts rose by about 200 in each study group. The groups did not differ in changes in inflammatory biomarkers, lipids, glucose, or weight. Almost one quarter of participants in each arm had a grade 3 or 4 adverse event. One person taking vitamin D and calcium and 2 taking placebo discontinued treatment because of toxicity. Nephrolithiasis developed in 1 person taking placebo, and no cases of hypercalcemia emerged.
The ACTG team characterized this supplementation strategy as a "low-cost intervention to prevent bone loss with low toxicity." During the question-and-answer session after the presentation attendees wondered whether a lower vitamin D dose may be effective (especially for people with moderate vitamin D deficits), whether calcium might be dropped from the regimen in light of its potentially negative cardiovascular impact [2], and whether the intervention makes sense for people taking regimens not including tenofovir or efavirenz.
link to webcast:
http://www.croiwebcasts.org/console/player/22267?mediaType=slideVideo&
References
1. Overton ET, Chan ES, Brown TT, et al. High-dose vitamin D and calcium attenuates bone loss with ART initiation: results from ACTG A5280. CROI 2014. Conference on Retroviruses and Opportunistic Infections. March 3-6, 2014. Boston. Abstract 133.
2. Bolland MJ1, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. http://www.bmj.com/content/342/bmj.d2040?view=long&pmid=21505219
----------------------------------------
High-Dose Vitamin D and Calcium Attenuates Bone Loss with ART Initiation: Results from ACTG A5280
A Prospective, Randomized Pilot Trial of High-Dose Vitamin D and Calcium for Bone Health in HIV-Infected Individuals Initiating Highly Active Antiretroviral Therapy
Reported by Jules Levin
CROI 2014 March 3-6 Boston, MA
Overton ET, Chan ES, Brown TT, Tebas P, McComsey GA, Melbourne K, Napoli A, Hardin R, Ribaudo HJ, Yin MT for the A5280 Study Team.
"our data only addresses the utility of supplementation during the first year of starting EFV/TDF/FTC. We don't have data to show whether it is beneficial beyond one year. I suspect that the majority of the benefit will occur within the first year, so the patient/doctor discussion should be focused only upon VitD and calcium supplementation in that time period."
|
| |
|
 |
 |
|
|