 |
 |
 |
| |
Estimating Health Status Using EQ-5D for Chronic Hepatitis C (CH-C) Patients Treated with Sofosbuvir Containing Regimens
|
| |
| |
Reported by Jules Levin
EASL 2014 April 9-13 London, UK
Zobair M. Younossi, Maria Stepanova, Sandrine Cure, Francois Bourrhis, Fatema H. Nader, Sharon L. Hunt
1.Betty and Guy Beatty Center for Integrated Research, Inova Health System, Falls Church VA, United States.
2.Center for Liver Diseases, Department of Medicine, Inova Fairfax Medical Campus, Falls Church, VA, United States.
3. OptumInsight UK, Uxbridge, United Kingdom.
4. OptumInsight France, Nanterre, France.
added to this report by Jules: {Established in 1987, the EuroQol Group initially comprised a network of international, multilingual and multidisciplinary researchers from seven centres in Finland, the Netherlands, Norway, Sweden and the UK. Nowadays, the Group comprises researchers from Canada, Denmark, Germany, Greece, Japan, New Zealand, Slovenia, Spain, the USA and Zimbabwe. The process of shared development and local experimentation resulted in EQ-5D, a generic measure of health status that provides a simple descriptive profile and a single index value that can be used in the clinical and economic evaluation of health care and in population health surveys. Currently, EQ-5D is being widely used in different countries by clinical researchers in a variety of clinical areas. EQ-5D is also being used by eight out of the first 10 of the top 50 pharmaceutical companies listed in the annual report of Pharma Business (November/December 1999). Furthermore, EQ-5D is one of the handful of measures recommended for use in cost-effectiveness analyses by the Washington Panel on Cost Effectiveness in Health and Medicine. EQ-5D has now been translated into most major languages with the EuroQol Group closely monitoring the process.
The EQ-5D includes single item measures of: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each item is coded using 3-levels (1 = no problems; 2 = some problems; 3 = severe problems). The instrument includes a global rating of current health using a visual analog scale (VAS) ranging from 0 (worst imaginable) to 100 (best imaginable). An additional single item measure of health change (better, much the same, worse) was included.}
Program abstract-
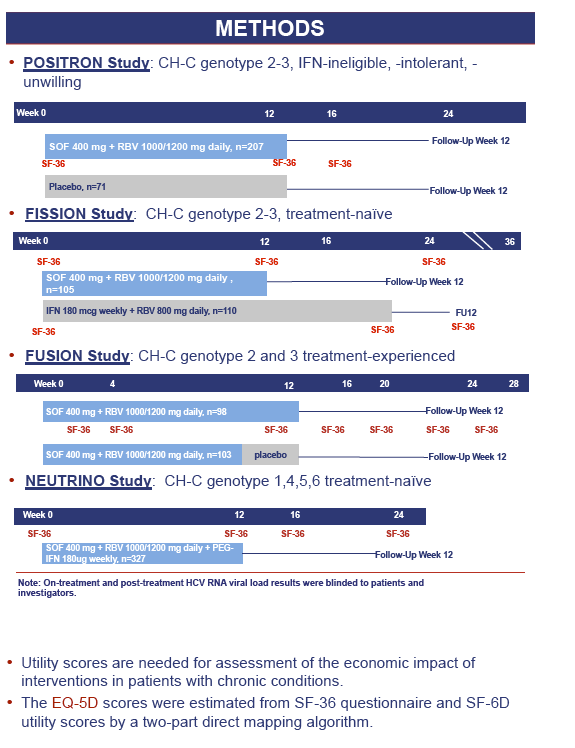
Background and aims: Health status assessment with EQ5D is important for economic analysis and clinical trials. We estimated EQ5D scores for patients receiving SOF+ribavirin (RBV) with or without peg-interferon (PEG-IFN).
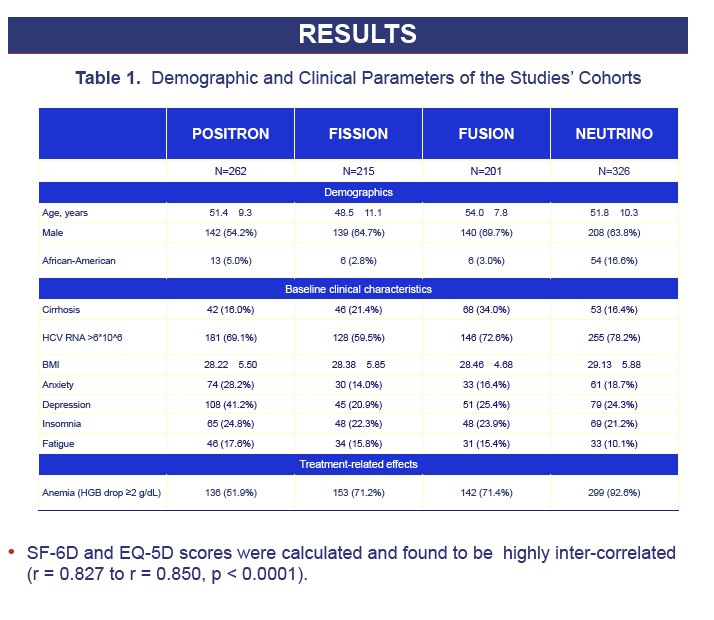
Methods: Using Short Form-36 (SF-36) and SF6D, we calculated EQ5D scores (Gray 2004) by a two-part direct mapping algorithm (baseline, end-of-treatment and post-treatment).
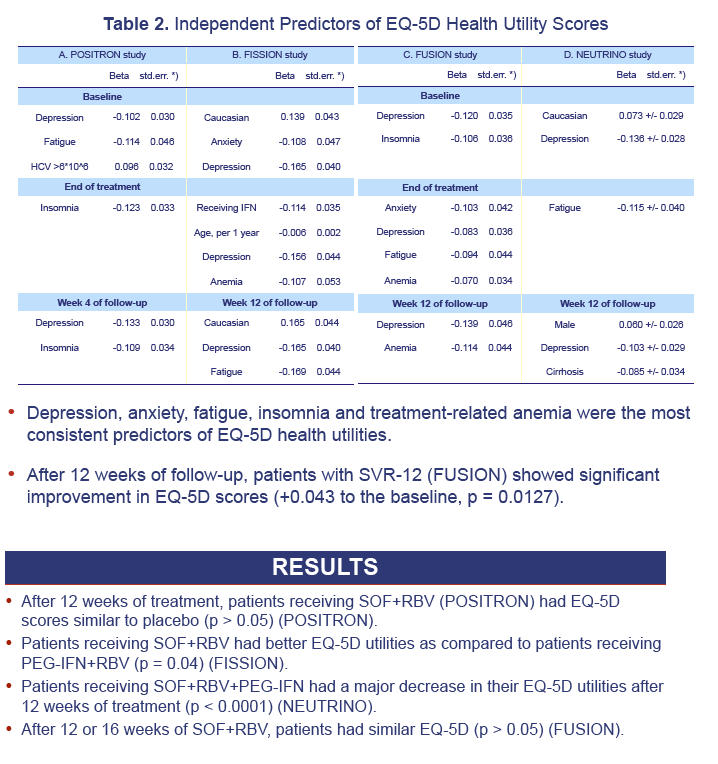
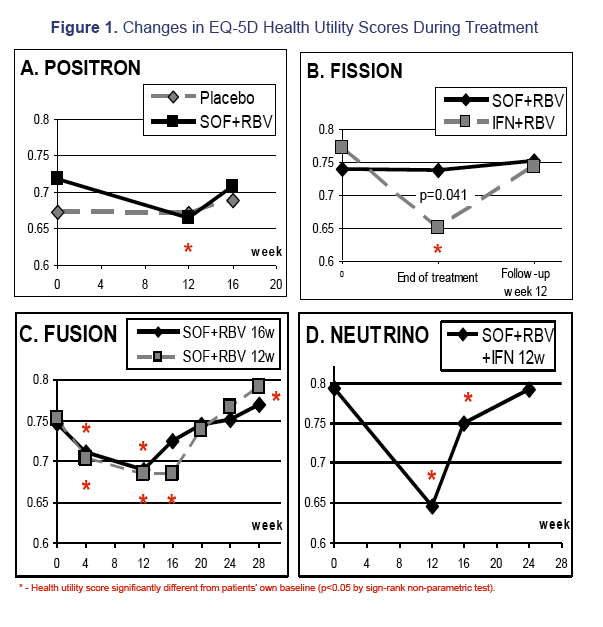
Results: 1,004 CH-C patients with SF-36 data were included [POSITRON (N=262), FISSION (N=215), FUSION (N=201), NEUTRINO (N=326)]. SF6D and EQ5D scores were calculated and highly inter-correlated (r=0.71 to r=1.0, p< 0.0001). Baseline EQ5D scores were 0.706±0.218 (POSITRON), 0.755±0.234 (FISSION), 0.749±0.221 (FUSION) and 0.793±0.219 (NEUTRINO). After treatment week 12, patients receiving SOF+RBV (POSITRON) had similar EQ5D scores to placebo (p>0.05). Furthermore, patients receiving 12 or 16 weeks of SOF+RBV had similar EQ5D scores (FUSION) (p>0.05). In FISSION, patients receiving PEG-IFN+RBV had substantially lower EQ5D scores compared to SOF+RBV (0.650±0.224 vs. 0.737±0.251, p=0.041). Patients receiving PEG-IFN+SOF+RBV (NEUTRINO) had a major decrease in their EQ5D scores during treatment (from 0.793±0.219 to 0.645±0.213, p< 0.0001) similar to scores observed in patients receiving PEG-IFN+RBV (FISSION) (from 0.771±0.236 to 0.650±0.224, p< 0.0001). After 12 weeks of follow-up, patients with SVR-12 (FUSION) showed significant improvement in EQ5D scores (+0.043 to the baseline, p=0.0127). In multivariate analyses, baseline depression, anxiety, fatigue, insomnia, treatment-related anemia and receiving IFN were predictors (p< 0.001) of lower EQ5D scores.
Conclusions: This is the first study estimating EQ5D scores from SF-36 in CH-C. EQ5D scores are minimally impacted by IFN-free SOF regimens. These patients had significantly higher EQ5D scores than those receiving IFN-containing regimens.
|
| |
|
 |
 |
|
|