| |
NS5A Samatasvir(IDX719) Monotherapy in Gt1/2/3/4
|
| |
| |
presented as a poster: American Association for the Study of Liver Diseases; Boston, MA; November 9-13, 2012
IDX719, HCV NS5A Inhibitor, Demonstrates Pan-Genotypic Activity after Three Days of Monotherapy in Genotype 1, 2, 3 or 4 HCV-Infected Subjects
http://www.natap.org/2013/HCV/013113_03.htm
Janssen / Idenix Pharmaceuticals Announces Initiation of Enrollment in a Phase II All-Oral Combination Study of Samatasvir (IDX719), Simeprevir, and TMC647055 for the Treatment of Hepatitis C Virus (HCV) Infection......Dec. 2, 2013
http://www.natap.org/2013/HCV/120313_01.htm
Idenix Pharmaceuticals Reports Sustained Virologic Response Rate (SVR4) for Phase II All-Oral Combination Study of Samatasvir (IDX719), a Potent, Pan-Genotypic HCV NS5A Inhibitor, and Simeprevir
http://www.natap.org/2013/HCV/011314_01.htm
Idenix Nucleotide/NS5A Update.....IDX21437, a next-generation uridine nucleotide prodrug inhibitor
http://www.natap.org/2013/HCV/103113_01.htm
Study Assessing Single and Multiple Doses of IDX21437 in Healthy and HCV-Infected Subjects.....Phase I/IIa Study Assessing Single and Multiple Doses of IDX21437 in Healthy and HCV-Infected
Subjects......http://clinicaltrials.gov/show/NCT01974687
Review at HepDart:
See attached pdf
Download the PDF here
Download the PDF here
------------------------------------
Article in Press
A randomized, double-blind, multiple-dose study of the pan-genotypic NS5A inhibitor samatasvir in patients infected with hepatitis C virus genotype 1, 2, 3 or 4
Journal of Hepatology
Bradley Vince, John M. Hill, Eric J. Lawitz, William O'Riordan, Lynn R. Webster, Daniel M. Gruener, Ricky S. Mofsen, Abel Murillo, Eile
en Donovan, Jie Chen, Joseph F. McCarville, John Z. Sullivan-Bolyai, Douglas Mayers, Xiao-Jian Zhou
Abstract
Background & Aims
Samatasvir is a pan-genotypic inhibitor of the hepatitis C (HCV) nonstructural protein 5A (NS5A). This study evaluated the antiviral activity, pharmacokinetics and safety of samatasvir monotherapy in treatment-naïve subjects infected with HCV genotype 1-4.
Methods
Thirty-four genotype 1 and thirty genotype 2, 3 or 4 subjects were randomized to receive for 3 days placebo or samatasvir 25-100 mg per day. Plasma samples for HCV RNA, pharmacokinetics and sequencing were collected up to day 10.
Results
Samatasvir achieved potent antiviral activity across genotypes: mean maximum reductions from baseline were 3.2-3.6 (genotype 1a), 3.0-4.3 (genotype 1b), 3.2-3.4 (genotype 3) and 3.6-3.9 (genotype 4) log10/mL respectively; no viral rebound was observed during the 3-day treatment period. For genotype 2 HCV, samatasvir was active in subjects with NS5A L31 polymorphism at baseline (individual range 2.5-4.1 log10/mL), but showed minimal activity in those with baseline M31 polymorphism. Samatasvir exhibited a long plasma half-life of approximately 20 hours which supports once daily dosing. Samatasvir was well tolerated in all subjects with no safety-related discontinuations or serious adverse events. The most common adverse events included constipation, nausea and headache and occurred at similar frequency in active and placebo subjects. All events were mild or moderate in intensity. There were no patterns or dose dependence of adverse events, vital signs, laboratory parameters or electrocardiograms.
Conclusions
Samatasvir 25-100 mg monotherapy for 3 days was well tolerated and induced a rapid and profound reduction in plasma HCV RNA in subjects infected with HCV genotype 1-4. Samatasvir is being evaluated in combination with other direct-acting antiviral agents in subjects with HCV infection.
EXCERPTS
Samatasvir (IDX719), a novel NS5A inhibitor of HCV replication, exhibits potent and pan-genotypic anti-HCV activity with in vitro 50% effective concentration (EC50) values ranging from 2 to 24 pM against HCV of genotypes 1a, 1b, 2a, 3a, 4a and 5a. There is only a 12-fold shift in EC50 values from the most sensitive genotype 4a to the least sensitive genotype 2a. With a 50% cytotoxicity concentration >50 μM, samatasvir has a high selectivity index of at least 2,000,000 [7,8]. Fig. 1 illustrates the chemical structure of samatasvir.
Samatasvir was evaluated in a two-part clinical study. Part one included single dose escalation and repeat dose administration in healthy subjects and an exploratory single-dose administration in subjects infected with HCV genotype 1, 2 or 3. Results from part one, reported elsewhere, showed that single and repeat doses of samatasvir up to 100 mg in healthy volunteers and single doses up to 100 mg in 99 HCV-infected subjects were well-tolerated and achieved pharmacologically relevant drug exposure. Samatasvir exhibited dose-proportional plasma exposure and long plasma half-life, supporting once daily (QD) dosing [5]. Single doses of samatasvir demonstrated substantial pan-genotypic antiviral activity of up to 3.7 log10 IU/mL in patients with genotype 1, 2 or 3 HCV [5].
Part two of the study, reported here, evaluated the safety, pharmacokinetics (PK) and antiviral activity of samatasvir as a single agent following multiple doses up to 100 mg daily for 3 days in subjects infected with HCV genotype 1, 2, 3 or 4.
This was a multicenter, randomized, double-blind, placebo-controlled, parallel-panel, multiple-dose study of samatasvir as a single agent dosed for 3 days in treatment-naïve patients with chronic HCV genotype 1, 2, 3 or 4. Thirty-four patients with genotype 1 HCV were randomized to receive either samatasvir (n=28) or placebo (n=6): 25 mg and 50 mg QD cohorts each had 8 active and 2 placebo subjects; 50 mg twice daily (BID) and 100 mg QD cohorts each had 6 active and 1 placebo subjects. Thirty subjects with HCV genotype 2, 3 or 4 were randomized to receive samatasvir 50 mg BID (n=12), 100 mg QD (n=12) or placebo (n=6) in an active-to-placebo ratio of 4:1 (ClinicalTrials.gov Identifier: NCT01508156). Treatment was assigned via a computer-generated randomization code and kept blinded to subjects and clinical investigators. Subjects were admitted to one of the 8 clinical sites in the United States between January 3, 2012 and July 9, 2012 and were required to stay in the clinical facility 120 from day -1 to study discharge on day 10 or upon early termination. Samatasvir oral suspension or matching placebo was administered under fasting conditions. Cohorts were dosed in parallel without dose escalation.
Antiviral activity
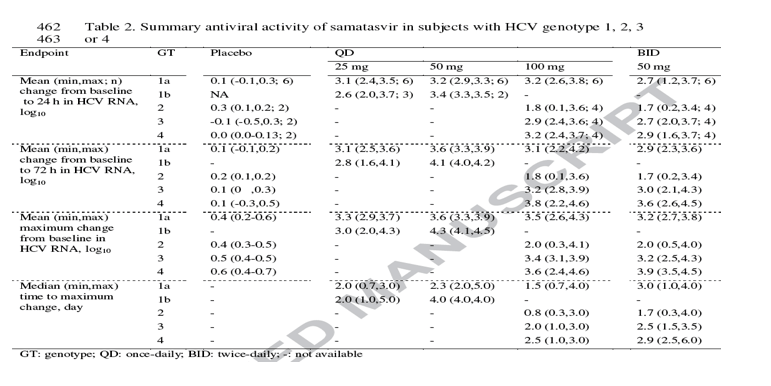
Mean changes over time of plasma HCV RNA from baseline are shown in Fig. 2 for subjects with genotype 1, 3 or 4 HCV. Fig 3 depicts the individual and mean changes over time of plasma HCV RNA from baseline for subjects with genotype 2 HCV. The mean change in log10 HCV RNA from baseline to 24 h and 72 h, the mean maximum change from baseline and the corresponding time were summarized in Table 2. Samatasvir dosed QD or BID for 3 days produced substantial pan-genotypic antiviral activity. The greatest antiviral activity was achieved in subjects having genotype 1b HCV with mean maximum decrease in HCV RNA of 3.0-4.3 log10, followed by 3.6-3.9 log10 in genotype 4, 3.2-3.6 log10 in genotype 1a and 3.2-3.4 log10 in genotype 3 HCV (see below for antiviral response in subjects with genotype 2 HCV). Maximum decrease in HCV RNA typically occurred upon completion of dosing. Antiviral activity was not observed in subjects receiving placebo. After completion of samatasvir dosing, plasma HCV RNA slowly returned towards baseline but did not attain pre-treatment level within the follow-up period of up to 10 days.
Antiviral response to samatasvir in subjects with 247 genotype 2 HCV was determined by a single polymorphism at the NS5A amino acid position 31. Among the 8 subjects with genotype 2 HCV who received samatasvir, high antiviral activity with maximum decrease in HCV RNA of 4.0 and 4.1 log10 was achieved in 2 subjects (Fig. 3, 002-006 and 004-019) who had L31 at baseline with no detectable M31 on day 4. In 2 subjects who had L31 at baseline but emergence of M31on day 4, robust antiviral activity was retained with maximum decrease in HCV RNA of 2.5 and 3.2 log10 (Fig. 3, 001-147 and 001-163). In 1 subject who had an L/M31 mixture at baseline but M31 on day 4, a much reduced antiviral activity (a decrease of 0.8 log10) was obtained (Fig. 3, 007-004). Virtually no antiviral activity (0.3-0.5 log10 reduction) was observed in the 3 subjects with pre-existing M31 who received samatasvir (Fig. 3, 001-188, 002-017 and 002-016). In contrast, despite all genotype 4 -infected subjects carrying an NS5A M31 at baseline, all responded well to samatasvir treatment (Fig. 2). Additional details on sequence analyses of other studied HCV genotypes will be reported elsewhere.
Predictors of antiviral response
Among various baseline characteristics and dose examined using regression analysis, only genotype and dose were significant predictors of viral response (P < 0.0001). Genotype 1b was the most susceptible following by 1a, 3 and 4 which responded comparably to samatasvir; genotype 2 was the least susceptible due to high prevalence of the pre-existing M31 or M/L31 polymorphisms which virtually did not respond to samatasvir.
Safety and tolerability
Samatasvir was well-tolerated in all subjects. There were no treatment-emergent deaths, SAEs or safety-related discontinuations. AEs were reported at a similar frequency in samatasvir-treated subjects (20 of 48 or 41.7%) and placebo (6 of 12 or 50.0%). As summarized in Table 4, the most frequent AEs reported were constipation, headache, and nausea. All AEs were mild or moderate in intensity and did not appear to be dose related. There were no apparent dose-related or other patterns of newly 290 occurring or worsening graded hematology, chemistry, or urinalysis abnormalities. There were no clinically significant treatment-emergent changes in vital sign measurements, physical examination findings or ECG parameters.
Discussion
Results from the current 3-day proof-of-concept part of the study confirmed the pan-genotypic antiviral activity observed during the exploratory single-dose phase, but showed more profound and persistent virologic response due to continuous suppressive pressure resulting from multiple doses. Subjects with genotype 1 HCV achieved mean maximum reduction of plasma HCV RNA of 3.0-4.3 log10, which is numerically in the upper 2.3-4.0 log10 range of virologic response obtained with other clinical stage NS5A inhibitors as a single agent dosed for 1 to 14 days (median 3 days) [9-17] At the tested doses administered for 3 days in subjects with genotype 3 or 4 HCV, virology responses were comparable with those observed in genotype 1 subjects with a mean maximum reduction of 3.2-3.9 log10. A similar degree of viral suppression was also achieved in subjects with genotype 2 HCV who had no pre-existing M31 or L/M31 polymorphism. Pre-existing M31 polymorphism in genotype 2 subjects predicts lack of virology response, and emerging M31 is associated with reduced antiviral activity to samatasvir mono therapy.
While all current clinical-stage NS5A inhibitors are able to 334 induce substantial early viral response, this class of HCV DAA is, however, prone to rapidly select viral variants as a single agent [3]. Indeed, viruses in the current study underwent treatment emergent selection of NS5A variants associated with in vitro resistance (primarily at positions 93, 28, 30 and 31, details to be presented elsewhere) although no subject experienced an on-treatment rebound defined as a 0.5 log10 increase above nadir with samatasvir as a single agent dosed for 3 days. During a 14-day mono therapy with daclatasvir, viral rebounds occurred early (generally before 7 days) and were associated with emergence of resistant variants [4]. The lack of viral breakthrough while on samatasvir in the current study contrasts with the observed rapid selection of resistance associated variants. These conflicting observations might be a consequence of the short duration of treatment, i.e., the treatment-selected variants may not have had sufficient time to rebound from their suppressed levels in the presence of drug. The low barrier to resistance with NS5A inhibitors as monotherapy appears to have little clinical relevance when these drugs are used in combination with other classes of DAAs including nucleotide, non-nucleotide NS5B and protease inhibitors. In fact, the majority of the best sustained virologic response data (>90%) obtained to date are from experimental all-oral combination regimens involving NS5A inhibitors [18-21].
Samatasvir exhibited a consistent and long plasma half-life of 20 h across doses/regimens. Its long half-life is in the range of 12-16 h and 13-50 h reported respectively for daclatasvir and ledipasvir, the most advanced NS5A inhibitors in clinical development [9,10]. Long half-life results in sustained plasma exposures and supports QD dosing of samatasvir. Despite being able to achieve higher trough concentrations, for the same total daily dose of 100 mg, samatasvir dosed BID did not produce more virologic response than QD dosing, presumably due to the fact that both regimens resulted in troughs largely surpassing the protein-binding adjusted 90% effective concentration (EC90) of the drug against the tested HCV genotypes. Albeit limited number of subjects for each (sub)-genotype per cohort and rather comparable viral declines across most genotypes, a multivariate analysis was able to identify HCV genotype and dose as the only predictors of antiviral response. In this 3-day trial with samatasvir as a single agent, subjects with genotype 1b HCV had the best response followed by genotype 1a, 3 or 4 with similar responses. Subjects with genotype-2 HCV having emerging M31, pre-existing M31 or M/L31 polymorphisms had reduced to no response to samatasvir. Therefore, the relative clinical potencies of samatasvir observed in the present study against various HCV genotypes were in good agreement with in vitro data [7,8]. Traditional predictors of viral response including baseline viral load and IL28B polymorphism were not significant predictors.
|
|
| |
| |
|
|
|