| |
European Commission Approves Bristol-Myers Squibb's Daklinza
(daclatasvir) Across Multiple Genotypes for
the Treatment of Chronic Hepatitis C Infection
|
| |
| |
Daklinza, when used in combination with sofosbuvir, is an all-oral, once daily regimen that yields cure rates of up to 100%
Daklinza + sofosbuvir offers potential cure for a broad range of EU HCV patients, including those with advanced liver disease, genotype 3 and protease inhibitor failures
Wednesday, August 27, 2014 5:00 am EDT
"We are proud to have discovered, developed and now brought to market this first-in-class NS5A replication complex inhibitor. We look forward to our continued work with EU health authorities to ensure Daklinza-based regimens are available to patients as quickly as possible."
PRINCETON, N.J.--(BUSINESS WIRE)--Bristol-Myers Squibb Company (NYSE:BMY) today announced that the European Commission has approved Daklinza (daclatasvir), a potent, pan-genotypic NS5A replication complex inhibitor (in vitro), for use in combination with other medicinal products across genotypes 1, 2, 3 and 4 for the treatment of chronic hepatitis C virus (HCV) infection in adults. Daklinza, when used in combination with sofosbuvir, is an all-oral, interferon-free regimen that provided cure rates of up to 100% in clinical trials, including patients with advanced liver disease, genotype 3 and those who have previously failed treatment with protease inhibitors. Daklinza is the first NS5A complex inhibitor approved in the European Union (EU) and will be available for use in combination with other medicinal products, providing a shorter treatment duration (12 or 24 weeks) compared to 48 weeks of treatment with interferon- and ribavirin-based regimens.
Today's approval allows for the marketing of Daklinza in all 28 Member States of the EU. The marketing authorization for Daklinza follows an accelerated assessment by the Committee for Medicinal Products for Human Use (CHMP), a designation that is granted to new medicines of major public health interest.
"HCV is a challenging virus to overcome, requiring multiple modes of attack. With the approval of Daklinza, we have a new class of drug that disrupts the virus in two ways - by inhibiting both viral replication and assembly - and when combined with other compounds often results in cure among even the hardest-to-treat patients," said Michael P. Manns, MD, Professor and Chairman, Department of Gastroenterology, Hepatology, and Endocrinology, Hannover Medical School, Hannover, Germany.
Of the estimated nine million people living with HCV in the EU, genotype 1 is the most common genotype, though distribution varies across the region. The burden of liver disease and other morbidities from HCV infection is significant in Europe, where HCV accounts for 63% of liver transplants among patients with virus-related liver disease. Patient populations with high unmet needs include those with advanced liver disease, protease inhibitor failure, genotype 3, HIV co-infected patients and those who have undergone liver transplant.
"The eradication of HCV is in sight, and with today's approval, Daklinza, in combination with other agents, will be an important option to achieve cure across many HCV genotypes and patient types for those in the EU who are in dire need of new treatment choices," said Emmanuel Blin, Head of Worldwide Commercialization, Bristol-Myers Squibb. "We are proud to have discovered, developed and now brought to market this first-in-class NS5A replication complex inhibitor. We look forward to our continued work with EU health authorities to ensure Daklinza-based regimens are available to patients as quickly as possible."
The approval of Daklinza is supported by data from multiple studies, including an open-label, randomized study of Daklinza with sofosbuvir in genotypes 1, 2, and 3, including patients with no response to prior therapy with telaprevir or boceprevir and patients with fibrosis. Results showed that a regimen of Daklinza with sofosbuvir achieved SVR12 (sustained virologic response 12 weeks after the end of treatment; a functional cure) in 99% of treatment-na•ve patients with HCV genotype 1, 100% of patients with genotype 1 who had failed treatment with either telaprevir or boceprevir, 96% of those with genotype 2 and 89% of those with genotype 3.
In addition, the regimen resulted in low rates of discontinuation (<1%) due to adverse events (AEs). The rate of serious adverse events (SAEs) was low (4.7%).
The most common adverse events were fatigue, headache and nausea. Across clinical studies, Daklinza-based regimens have been generally well tolerated with low rates of discontinuation across a range of patients. Ongoing and completed Daklinza studies have included more than 5,500 patients in a variety of all-oral regimens and with the current interferon-based standard of care.
The safety of Daklinza for the treatment of hepatitis C has been demonstrated in diverse patient populations that include elderly patients, patients with advanced liver disease, post-liver transplant recipients and patients co-infected with HIV. No unique safety concerns have been identified in patients who were treated with Daklinza across clinical studies and in the early access program. Several of these studies are ongoing.
Recommended regimens and treatment duration for Daklinza combination therapy include:
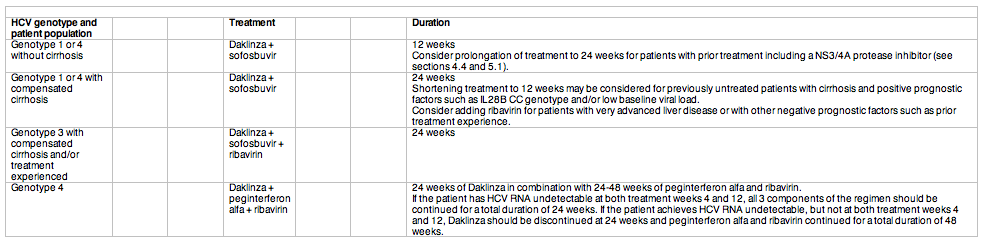
Daklinza monotherapy is not recommended. The Summary of Product Characteristics will be available at www.ema.europa.eu. Commercial availability of Daklinza in the EU will be determined by individual Member States.
About Hepatitis C
Globally, there are 150 million people infected with HCV and of that, an estimated 9 million people are living with hepatitis C in the European Union (EU). Hepatitis C is a virus that infects the liver and is transmitted through direct contact with infected blood and blood products. Up to 90 percent of those infected with hepatitis C will not spontaneously clear the virus and will become chronically infected. According to the World Health Organization, 20 percent of people with chronic hepatitis C will develop cirrhosis and, of those, about 5 to 7 percent of patients may ultimately die of the consequences of infection.
About Bristol-Myers Squibb's HCV Portfolio
Bristol-Myers Squibb's research efforts are focused on advancing late-stage compounds to deliver the most value to patients with hepatitis C. At the core of our pipeline is daclatasvir, a potent pan-genotypic NS5A complex inhibitor (in vitro), which continues to be investigated in multiple treatment regimens and in people with co-morbidities.
Daklinza was recently approved in Japan in combination with Sunvepra (asunaprevir), a NS3/4A protease inhibitor. The Daklinza+Sunvepra Dual Regimen is Japan's first all-oral, interferon- and ribavirin-free treatment regimen for patients with genotype 1 chronic HCV infection, including those with compensated cirrhosis.
Applications for the daclatasvir Dual Regimen are also under review by the U.S. Food and Drug Administration (FDA), which granted priority review status and set a target review date under the Prescription Drug User Fee Act (PDUFA) of November 30, 2014.
In 2014, the FDA granted Bristol-Myers Squibb's investigational daclatasvir Dual Regimen (daclatasvir + asunaprevir) Breakthrough Therapy Designation for use as a combination therapy in the treatment of genotype 1b HCV infection.
In 2013, Bristol-Myers Squibb's investigational all-oral 3DAA Regimen (daclatasvir/asunaprevir/BMS-791325) also received Breakthrough Therapy Designation in the U.S., which helped to expedite the start of the ongoing Phase 3 UNITY Program. Study populations include non-cirrhotic na•ve, cirrhotic na•ve and previously treated patients. The daclatasvir 3DAA Regimen is being studied as a fixed-dose-combination treatment with twice daily dosing.
Additional studies with daclatasvir in combination with sofosbuvir are being conducted in high unmet need patients, such as pre- and post-transplant patients, HIV/HCV co-infected patients and patients with genotype 3 as part of the ongoing Phase 3 ALLY Program.
About Bristol-Myers Squibb
Bristol-Myers Squibb is a global biopharmaceutical company whose mission is to discover, develop and deliver innovative medicines that help patients prevail over serious diseases. For more information, please visit http://www.bms.com or follow us on Twitter at http://twitter.com/bmsnews.
Bristol-Myers Squibb Forward Looking Statement
This press release contains "forward-looking statements" as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding the research, development and commercialization of pharmaceutical products. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including factors that could delay, divert or change any of them, and could cause actual outcomes and results to differ materially from current expectations. No forward-looking statement can be guaranteed. Among other risks, there can be no guarantee that Daklinza will be a commercially successful product. Forward-looking statements in this press release should be evaluated together with the many uncertainties that affect Bristol-Myers Squibb's business, particularly those identified in the cautionary factors discussion in Bristol-Myers Squibb's Annual Report on Form 10-K for the year ended December 31, 2013, in our Quarterly Reports on Form 10-Q and our Current Reports on Form 8-K. Bristol-Myers Squibb undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise.
Contact:
Bristol-Myers Squibb Company
Media:
Carrie Fernandez
Office: +1 609-252-4831
Cell: +1 215-859-2605
carrie.fernandez@bms.com
or
Jeff Smith
Office: +33(0)1 58 83 83 21
Cell: +33(0)6 03 99 40 18
JR.Smith.paeurope@bms.com
or
Investors:
Ranya Dajani, +1 609-252-5330
ranya.dajani@bms.com
o
or
Ryan Asay, +1 609-252-5020
ryan.asay@bms.com
|
|
| |
| |
|
|
|