| |
Transient elastography: a new surrogate marker
of liver fibrosis influenced by major changes of transaminases
|
| |
| |
Download the PDF here
Journal of Viral Hepatitis May 2007
B. Coco,1 F. Oliveri,1 A. M. Maina,1 P. Ciccorossi,1 R. Sacco,1 P. Colombatto,1 F. Bonino2 and
M. R. Brunetto1 1UO Gastroenterologia ed Epatologia Ospedaliera, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy; and 2Universita di
Pisa, Direzione Scientifica della Fondazione IRCCS Policlinico, Milan, Italy
"In conclusion, transient elastography as measured by FibroScan® is an easy-to-perform, reproducible method that allows the rapid and objective evaluation of liver stiffness. In clinical practice, it is a reliable, noninvasive, surrogate marker of liver fibrosis that warrants an accurate, noninvasive staging of liver disease and may reduce the number of invasive liver biopsies for clinical decision making. However, liver stiffness represents a new liver parameter that differs from liver fibrosis, as it is influenced significantly by major variations of the biochemical activity of liver disease. Thus, in clinical practice, each liver stiffness measure has to be interpreted taking into account the concurrent biochemical profile of the patient. Future studies should analyse prospectively whether the amelioration of transient elastography correlates with changes of histological staging in sustained responders to antiviral therapy and clarify the relations between the liver stiffness and the type and extent of intrahepatic necroinflammation."
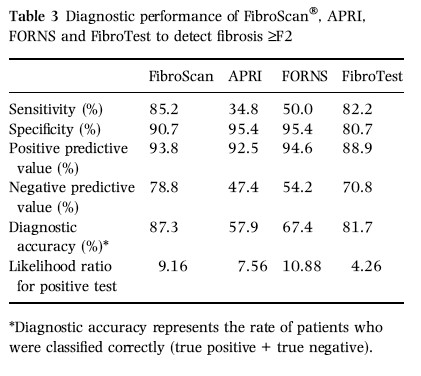
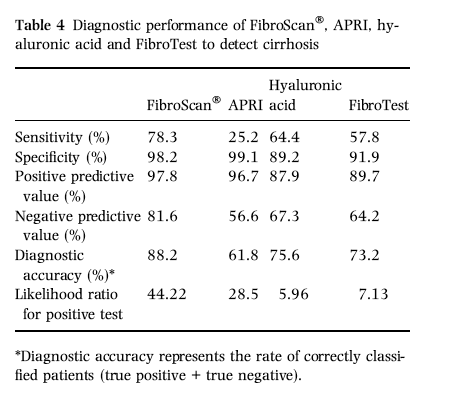
"In our hands, FibroScan® showed higher diagnostic accuracy than other noninvasive surrogate markers of liver fibrosis. We found that FT at the recommended cut-off had lower PPV and NPV when compared with the original report (88.9% and 70.8%vs 91% and 100% for fibrosis ≥F2; 89.7% and 64.2%vs 85% and 90% for cirrhosis, respectively) [21]. Similar observations were reported by Rossi et al. [24]. Castera et al. using different combinations of APRI, FT and FibroScan® showed that FibroScan® and FT together had the best diagnostic performance for diagnosis of both fibrosis and cirrhosis [30]. At variance with Castera et al. [30], we did not find that the combination of different assays ameliorate the accuracy of FibroScan® with the exception of the subgroup of cirrhotics whose disease was in prolonged biochemical remission."
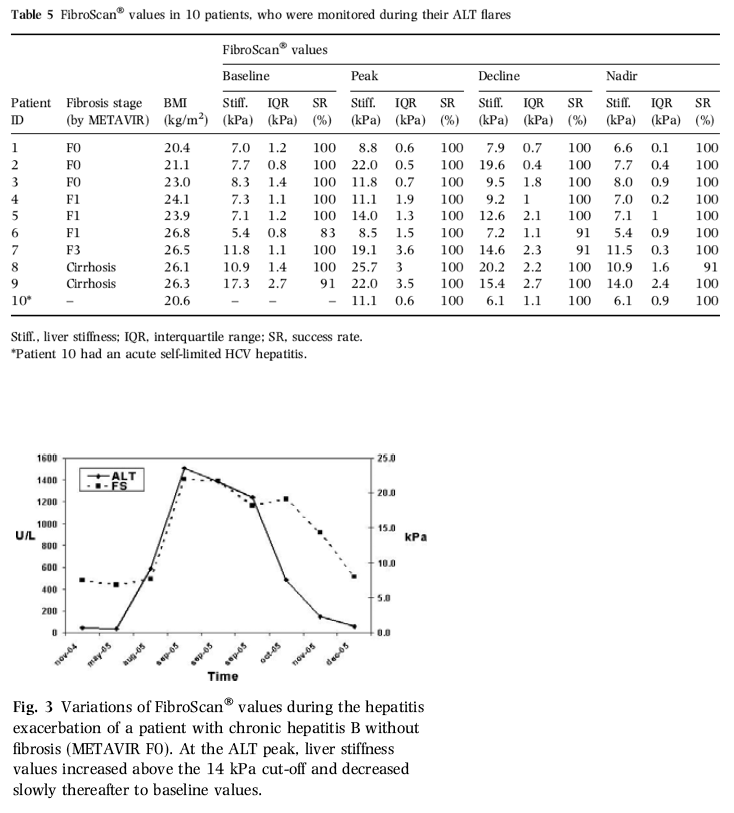
"Our 14 kPa cut-off caused the overestimation of cirrhosis in two patients only, who had F3 fibrosis at histology. On the contrary, the specificity for diagnosis of fibrosis ≥F2 was slightly worse and fibrosis was overestimated in eight cases (9.3%). However, two of these patients were examined during their hepatitis exacerbations, and we found that ALT as well as fibrosis was independently associated with liver stiffness at multivariate analysis. During tight monitoring, the liver stiffness profile of the patients with ALT flares differed significantly from that of the patients with elevated ALT, but without flares (P = 0.001). Accordingly, in 10 patients with acute exacerbations, liver stiffness increased 1.3- to 3-fold at the time of their ALT flares and declined progressively to baseline thereafter. These observations suggest that transient elastography is influenced by major changes of the biochemical profile such as hepatitis exacerbations or long-term remissions. In the majority of the patients with these features, fibrosis would have been overestimated if evaluated by a single stiffness determination only (Table 5). On the contrary, in patients with stable biochemical activity, liver stiffness values did not change significantly confirming the reliability of the technique in such conditions."
"The influence of biochemical activity on liver stiffness was evident also in the subset of patients with F0-F1 fibrosis and long-lasting biochemical remission in whom transient elastography was significantly lower (5.6 ± 1.8 kPa) than in patients with the same histological stages, but elevated ALT (6.6 ± 1.8 kPa) (P = 0.029). Similarly, biochemical remission resulted an independent factor influencing liver stiffness values (B = -0.250, 95% CI: -0.345 to -0.155, P < 0.001) in cirrhotic patients. Liver stiffness was lower in cirrhotics with biochemical remission than in cirrhotics with elevated ALT (14.9 ± 10.3 vs 25.6 ± 12.9 kPa, P < 0.001) and it was lower than 14 kPa in 13 of 25 with remission: 10 of these maintained US signs of cirrhosis in spite of low liver stiffness and the remaining three persistent cirrhosis at their more recent liver biopsies."
....The higher prevalence of biochemical remission in HBV (17 of 41) when compared with HCV cirrhotics (8 of 74) explains why liver stiffness was lower in the former group (19.2 and 28.6 kPa in HBV and HCV, respectively, Fig. 1b)."
-------------
Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases
Journal of Viral Hepatitis
May 2007
Abstract
Summary. Liver stiffness was measured by transient elastography (FibroScan®) in 228 consecutive patients with chronic viral hepatitis, with (115) or without cirrhosis (113), to study its correlations with serum transaminases [alanine aminotransferase (ALT)], fibrosis stage and surrogate noninvasive markers of fibrosis (APRI, FORNS, FibroTest and hyaluronic acid). The dynamic profiles of serum transaminases and liver stiffness were compared by multiple testing in 31 patients during a 6-month follow-up. We identified 8.3 and 14 kPa as the fibrosis ≥F2 and cirrhosis cut-offs, respectively: their sensitivities were 85.2%/78.3%; specificities 90.7%/98.2%; positive predictive values 93.9%/97.8%; negative predictive values 78.8%/81.6%; diagnostic accuracies 87.3%/88.2%. FibroScan® performed better than the other surrogate markers of fibrosis (P < 0.001). Other than fibrosis, other factors independently associated with liver stiffness were ALT for all patients and chronic hepatitis patients (P < 0.001), and 12-month persistently normal ALT (biochemical remission, P < 0.001) in cirrhotics. In patients with biochemical remission either spontaneous or after antiviral therapy (48 of 228, 21%), liver stiffness was lower than in patients with identical fibrosis stage, but elevated ALT (P < 0.001). The liver stiffness dynamic profiles paralleled those of ALT, increasing 1.3- to 3-fold during ALT flares in 10 patients with hepatitis exacerbations. Liver stiffness remained unchanged in 21 with stable biochemical activity (P = 0.001). In conclusion, transient elastography is a new liver parameter that behaves as a reliable surrogate marker of fibrosis in chronic viral hepatitis patients, provided that its relationship with major changes of biochemical activity is taken into account.
Introduction
Histological staging is recommended by the American and European Associations for the Study of Liver Diseases to identify patients at risk of progressive liver disease and eligible to antiviral therapy [1-3]. However, liver biopsy is an invasive procedure, unsuitable for tight monitoring [4-6]. In addition, fragmented or small-sized specimens may cause the underestimation of fibrosis [7-11]. Several serum assays were proposed as surrogate markers of fibrosis [12-15], but only hyaluronic acid (HA) showed promising results as single parameters for the noninvasive assessment of cirrhosis [16-18]. A good accuracy to distinguish mild fibrosis from cirrhosis was achieved by algorithms using multiple measures, such as APRI, FORNS or FibroTest (FT) [19-23]. However, their performances worsened because of interlaboratory differences when such scores were applied in independent studies [24-26].
More recently, liver stiffness, measured by the speed of transmission of an elastic wave across the liver (transient elastography), was proposed as surrogate marker of fibrosis with high accuracy to identify cirrhosis [26-32].
We studied the clinical significance of transient elastography for the management of the patients with chronic hepatitis B (CHB) and C (CHC) with different biochemical profiles and compared its diagnostic performance to other surrogate markers of fibrosis.
Discussion
In the cohort of consecutive patients followed prospectively in our reference centre for chronic viral hepatitis, we confirmed that liver stiffness as measured by transient elastography is a reliable noninvasive surrogate marker of liver fibrosis (≥F2 or cirrhosis) with good reproducibility and low inter- and intraobserver variability. Using 8.3 and 14 kPa as the cut-off values for fibrosis ≥F2 and cirrhosis, respectively, we obtained 93.9% and 97.8% PPV, 90.7% and 98.2% specificities and 87.3% and 88.2% diagnostic accuracies, confirming that FibroScan® is a reliable method to assess fibrosis in patients with chronic viral hepatitis [26,29-31]. Our results appear slightly better than previously reported: 85%vs 56-67% as sensitivity for fibrosis ≥F2 and 98.2%vs 91-97% as specificity for cirrhosis [29-31]. This might be explained in part by our criteria for the selection of the cut-off values and the exclusion from the analysis of all liver specimens <1.5 cm or with less than 11 portal tracts (7/166, 4.2%) [9-11]. In addition, our study population had a higher prevalence of cirrhotics (50.5%) and a lower prevalence of patients with intermediate fibrosis (F2-F3; 11.8%) than other studies.
Our 14 kPa cut-off caused the overestimation of cirrhosis in two patients only, who had F3 fibrosis at histology. On the contrary, the specificity for diagnosis of fibrosis ≥F2 was slightly worse and fibrosis was overestimated in eight cases (9.3%). However, two of these patients were examined during their hepatitis exacerbations, and we found that ALT as well as fibrosis was independently associated with liver stiffness at multivariate analysis. During tight monitoring, the liver stiffness profile of the patients with ALT flares differed significantly from that of the patients with elevated ALT, but without flares (P = 0.001). Accordingly, in 10 patients with acute exacerbations, liver stiffness increased 1.3- to 3-fold at the time of their ALT flares and declined progressively to baseline thereafter. These observations suggest that transient elastography is influenced by major changes of the biochemical profile such as hepatitis exacerbations or long-term remissions. In the majority of the patients with these features, fibrosis would have been overestimated if evaluated by a single stiffness determination only (Table 5). On the contrary, in patients with stable biochemical activity, liver stiffness values did not change significantly confirming the reliability of the technique in such conditions.
The influence of biochemical activity on liver stiffness was evident also in the subset of patients with F0-F1 fibrosis and long-lasting biochemical remission in whom transient elastography was significantly lower (5.6 ± 1.8 kPa) than in patients with the same histological stages, but elevated ALT (6.6 ± 1.8 kPa) (P = 0.029). Similarly, biochemical remission resulted an independent factor influencing liver stiffness values (B = -0.250, 95% CI: -0.345 to -0.155, P < 0.001) in cirrhotic patients. Liver stiffness was lower in cirrhotics with biochemical remission than in cirrhotics with elevated ALT (14.9 ± 10.3 vs 25.6 ± 12.9 kPa, P < 0.001) and it was lower than 14 kPa in 13 of 25 with remission: 10 of these maintained US signs of cirrhosis in spite of low liver stiffness and the remaining three persistent cirrhosis at their more recent liver biopsies.
The higher prevalence of biochemical remission in HBV (17 of 41) when compared with HCV cirrhotics (8 of 74) explains why liver stiffness was lower in the former group (19.2 and 28.6 kPa in HBV and HCV, respectively, Fig. 1b).
These findings are consistent with the reports of Poynard et al., who showed the reduction of FT in HCV patients with sustained response to interferon and in HBV patients under long-term lamivudine treatment [35,36]. All these observations prompt future prospective studies where variations over time of noninvasive markers of fibrosis (such as liver stiffness or FT) should be correlated with histological grading and staging. These studies could answer the question whether the reduction of liver stiffness during prolonged biochemical remission is associated with the decrease of fibrosis. Furthermore, they should clarify whether liver stiffness depends not only on the building of molecular blocks of collagen and their structural organization (septa) but also on other factors such as the type and extent of the inflammatory infiltrate of the septa. In fact, our finding that liver stiffness increased during hepatitis exacerbations suggests that both inflammatory infiltrate and oedema might have significant impacts on the transient elastographic measure.
In our hands, FibroScan® showed higher diagnostic accuracy than other noninvasive surrogate markers of liver fibrosis. We found that FT at the recommended cut-off had lower PPV and NPV when compared with the original report (88.9% and 70.8%vs 91% and 100% for fibrosis ≥F2; 89.7% and 64.2%vs 85% and 90% for cirrhosis, respectively) [21]. Similar observations were reported by Rossi et al. [24]. Castera et al. using different combinations of APRI, FT and FibroScan® showed that FibroScan® and FT together had the best diagnostic performance for diagnosis of both fibrosis and cirrhosis [30]. At variance with Castera et al. [30], we did not find that the combination of different assays ameliorate the accuracy of FibroScan® with the exception of the subgroup of cirrhotics whose disease was in prolonged biochemical remission.
In conclusion, transient elastography as measured by FibroScan® is an easy-to-perform, reproducible method that allows the rapid and objective evaluation of liver stiffness. In clinical practice, it is a reliable, noninvasive, surrogate marker of liver fibrosis that warrants an accurate, noninvasive staging of liver disease and may reduce the number of invasive liver biopsies for clinical decision making. However, liver stiffness represents a new liver parameter that differs from liver fibrosis, as it is influenced significantly by major variations of the biochemical activity of liver disease. Thus, in clinical practice, each liver stiffness measure has to be interpreted taking into account the concurrent biochemical profile of the patient. Future studies should analyse prospectively whether the amelioration of transient elastography correlates with changes of histological staging in sustained responders to antiviral therapy and clarify the relations between the liver stiffness and the type and extent of intrahepatic necroinflammation.
Patients and Methods
Patients
We studied 241 consecutive CHB and CHC patients admitted at the Gastroenterology and Hepatology Unit of the University Hospital of Pisa (Italy) from April 2004 to April 2005. The study was approved by the Ethical Committee of the Hospital and the patients gave their written informed consent. Diagnosis of chronic hepatitis was based on the presence, at the time of the first observation, of active viral replication [serum HBV-DNA levels >105 copies/mL, by COBAS Amplicor HBV Monitor (Roche, Basel, Switzerland) and detectable HCV-RNA (COBAS Amplicor HCV Monitor, version 2.0; Roche or COBAS Amplicor HCV 2.0, sensitivity 50 IU/mL) in HBsAg and anti-HCV positive carriers, respectively] and liver histology consistent with chronic hepatitis. We excluded from the study: patients with Child B or C cirrhosis and patients under interferon or peg-interferon monotherapy or combination.
The histological diagnosis of liver disease was available from all the patients. Liver biopsies were performed within 6 months of the stiffness measurements (median 3 months; 75% of them performed between 0 and 3.8 months) in all the patients without ultrasound (US) signs of cirrhosis and within 3 years in patients with US signs of cirrhosis. Patients with cirrhosis underwent both liver US and Doppler examinations within 1 week from FibroScan®. Transaminases and liver function tests were determined in all the patients on the same day of FibroScan®. Serum apolipoprotein A1, haptoglobin, α-2 macroglobulin and HA were measured in 164 consecutive patients.
To evaluate the impact of biochemical activity on transient elastography, from January 2005 we proposed to monitor liver stiffness (on day 1, days 15, 30, 45, 60 and monthly for three additional months) to all the 15 patients who had a baseline FibroScan® evaluation and showed an alanine aminotransferase (ALT) flare thereafter and to 30 patients with persistent biochemical activity without flares. Overall, 30 patients (24 chronic hepatitis and 6 cirrhosis) accepted: 9 of them with hepatitis exacerbations. One additional patient with acute self-limited hepatitis C underwent serial measurements of liver stiffness.
Liver transient elastography
It was measured by FibroScan® (EchoSens, Paris, France), a noninvasive device based on a US transducer probe, mounted on the axis of a vibrator and linked to an electronic system. More details can be found elsewhere [27,28]. All measures were performed by two expert physicians (BC and FO) on the liver right lobes throughout intercostal spaces in patients lying on their back with right arms in maximal abduction. The US guide was used to identify a target liver area, at least 6 cm thick without major vascular structures. The procedure was based on at least 10 validated measurements: the success rate (ratio between numbers of validated and total measurements) was ≥60% with <20% interquartile ranges. Liver stiffness was recorded in kilopascals as the median value of all measurements [27,28].
Forty patients were randomly selected for both intra- and interobserver reproducibility: on the same patient, the former operator performed two series of measurements and the latter operator performed additional series of measurements blindly.
Noninvasive surrogate markers of fibrosis
Hyaluronic acid was measured by enzyme-linked binding protein assay (Corgenix, Westminster, CO, USA). APRI index, FORNS score and FT were calculated as previously reported [19,20,23]. For FT, we followed the recommendations of Biopredictive Lab (http://www.biopredictive.com) Biopredictive S.A.S., Paris, France [23].
Liver histology
Liver biopsies were obtained using 16 G disposable needles (Hepafix; B. Braun, Melsungen, Germany). Liver specimens (median 25 mm, range 12-54 mm) were stained with H&E. Necroinflammatory activity and liver fibrosis were scored according to Ishak [33] and METAVIR [34]. Steatosis was graded semiquantitatively, as reported [29]. Intrahepatic iron overload after Masson Trichrome and Perls staining was graded as absent, mild (+), moderate (++) and severe (+++). We excluded from the analysis all the specimens shorter than 1.5 cm and/or with less than 11 portal tracts.
Database
The variables were sex, age, aetiology (HBV and/or HCV) and liver disease cofactors [alcohol intake (< or >60 g/day), iron overload (present, in case of staining at histology and serum iron >150 mg/mL and/or ferritine >400 ng/mL), hyperlipidaemia (cholesterol >240 mg/dL and/or triglycerides >250 mg/dL), diabetes (fasting plasma glucose >140 mg/dL)] and overweight [body mass index (BMI) >25 kg/m2]. The biochemical profiles were defined as (a) persistently elevated ALT; (b) biochemical remission (persistently normal ALT for at least 12 months, at monthly controls) and (c) ALT flares (when ALT values increased ≥300 IU/L). The liver biopsy features were overall length; number of fragments; portal tracts number; necroinflammation and fibrosis scores; steatosis and/or iron overload scores. Cirrhosis at ultrasound (US cirrhosis) was defined when, in addition to histological diagnosis, US signs of cirrhosis (enlargement of left/caudate lobes; nodular liver boundaries; micro-macronodular liver structure) were present. We recorded in addition: the signs of portal hypertension (portal vein diameter >12 mm; spleen volume >45 cm2; oesophagus or gastric varices); the transient elastography performance (values; rate of successful measurements and interquartile ranges) and the characteristics of therapy (schedule, duration and response).
Statistical analysis
Statistical analysis was performed by SPSS (version 10.0, SPSS Inc., Chicago, IL, USA) and MEDCALC software packages. The logarithmic transformation was used for quantitative data when their distributions were not normal. Intra- and interobserver reproducibility was evaluated by Student's t-test for paired data. The Pearson's correlation coefficient was used to analyse the correlations between values of liver elastometry and fibrosis. Qualitative and quantitative differences between subgroups were analysed using corrected chi-square, Fisher's exact test and one-way ANOVA and Mann-Whitney rank sum test, respectively. Two-way ANOVA for repeated measures by GLM analysis was used to compare the dynamic profiles of liver elastometry between patients with ALT flares or stable biochemical activity.
The diagnostic performance of the tests was evaluated by receiver operating characteristic (ROC) curves. The area under the ROC curves (AUROCs) and 95% confidence intervals (CI) were used as indexes of accuracy and were compared by the Hanley and McNeil method. We defined two different cut-off values of liver transient elastography to identify patients with fibrosis ≥F2 (METAVIR score) or cirrhosis: for the former, we favoured the sensitivity to identify all the patients who need to be treated; for the latter the specificity to limit false-positive results. To identify factors independently correlated with liver stiffness, variables with statistical associations (P < 0.05) or trends (P < 0.10) at univariate were included in multiple regression analyses (forward stepwise method).
Results
Of 241 patients, 228 (94.6%) were suitable for the analysis (demographic and clinical characteristics in Table 1): 13 were excluded because their liver specimens were <1.5 cm or with <11 portal tracts [7] or elastographic measures failed (six cases; four with BMI >29). Fourteen patients with CHB (13 with cirrhosis confirmed by US signs and 1 with F0 fibrosis) were under nucleos(t)ide analogue treatment and in biochemical remission. Of 76 patients (28 with CHB and 48 with CHC) treated with interferon, peg-interferon or combination 12-36 months before their liver stiffness measure, 21 (17 with CHC) achieved a sustained virological response. In all these patients, a new liver biopsy was available after treatment.
Both intra- and interobserver variability were good (r = 0.964 and 0.916; Student's t-test for paired data: n.s.). Liver stiffness ranged between 3.8 and 75 kPa (median 15.2 kPa) and it was significantly correlated with fibrosis stage (r = 0.783, P < 0.001) independent of viral aetiology (Fig. 1a,b). At univariate analysis, liver stiffness correlated with age, alcohol intake >60 g/day, diabetes, ALT, biochemical remission, histological grading and fibrosis staging (Table 2). At multivariate analysis, ALT and fibrosis remained the only factors associated with liver stiffness (Table 2). After the separate analysis of the patients with or without cirrhosis, the factors independently associated with liver stiffness were ALT (B = 0.152, 95% CI: 0.082-0.222, P < 0.001) and fibrosis (B = 0.068, 95% CI: 0.042-0.095, P < 0.001) in chronic hepatitis without cirrhosis (Table 2) and biochemical remission (B = -0.250, 95% CI: -0.345 to -0.155, P < 0.001) and US signs of cirrhosis (B = 0.093, 95% CI: 0.013-0.174, P < 0.024) in cirrhotic patients (Table 2).
Area under the receiver operating characteristics for ≥F2 fibrosis and cirrhosis were of 0.932 and 0.957, respectively (95% CI: 0.902-0.963 and 0.934-0.980) (Fig. 2), and the cut-off values chosen to identify such conditions were 8.3 and 14 kPa, respectively.
Fibrosis ≥F2
The diagnostic performance of the 8.3 kPa cut-off for ≥F2 fibrosis is reported in Table 3. Overall, 121 of 129 patients with elastography ≥8.3 kPa had fibrosis ≥F2 (93.8% PPV), the remaining eight patients had lower fibrosis stages (four F0 and four F1): two of them had ALT flares at the time of examination. Of 99 patients with <8.3 kPa 78 (78.8% NPV) had F0-F1 fibrosis; of the remaining 21 patients, nine had fibrosis F2, four had F3, four had F4 and four had US cirrhosis. Five patients with cirrhosis and FibroScan values <8.3 kPa were in biochemical remission.
Cirrhosis
The diagnostic performance of the 14 kPa cut-off for cirrhosis is shown in Table 4. Of 92 patients with liver stiffness ≥14 kPa, 90 patients had histological or US signs of cirrhosis (97.8% PPV) and the remaining two patients had F3 fibrosis. Cirrhosis was absent in 111 of 136 patients with FibroScan® values <14 kPa; the remaining 25 were cirrhotics (81.6% NPV), 13 (52%) of them in biochemical remission.
Liver stiffness and biochemical activity
At the time of their elastometric measurements, 48 of 228 (21%) patients (23 with F0-F1 and 25 with cirrhosis) were in biochemical remission, either spontaneous (13 patients) or induced by therapy (35 patients). Their liver stiffness was lower than that in patients with comparable fibrosis, but elevated ALT: 5.6 (±1.8) kPa vs 6.6 (±1.8) kPa in patients with fibrosis < F2 (P = 0.029); 14.9 (± 10.3) kPa vs 25.6 (±12.9) kPa in cirrhotics (P < 0.001). Liver stiffness <14 kPa was found in 13 of 25 (52%) cirrhotics with biochemical remission and in 12 of 90 (13.3%) cirrhotics with elevated ALT (P < 0.001).
Two patients with F0-F1 fibrosis had ALT flares (8 and 15 x ALT normal values, respectively) at the time of transient elastography: both patients had FibroScan® values >8.3 kPa (8.8 and 11.1 kPa), which decreased below 8.3 kPa (6.5 and 7.0 kPa) after resolution of hepatitis reactivation.
Serial measurements of liver stiffness were performed during a mean follow-up of 8 months (6-18 months) in 31 patients: 21 patients with minor ALT fluctuations (< two fold) and 10 patients with ALT flares (one of them with acute hepatitis C). In patients with stable biochemical profiles (19 with hepatitis C and 2 with hepatitis B, mean BMI 23.2 kg/m2; fibrosis stage F0 in 7, F1 in 5, F2 in 2, F3 in 1, F4 in 5 and US cirrhosis in 1), liver stiffness showed minor variations (range -0.4- to 1.2-fold the baseline value), whereas it increased 1.3- to 3-fold during ALT flares and decreased to baseline values thereafter (Table 5; Fig. 3). Liver stiffness increased above the cut-off for fibrosis ≥F2 during hepatitis exacerbations in six patients with fibrosis F0-F1 and in one patient with acute hepatitis; one patient with fibrosis F3 showed values above the cut-off for cirrhosis (Table 5). Liver stiffness profiles were significantly different in patients with hepatitis flares when compared with those of patients with stable biochemical activities (P = 0.001).
Liver stiffness and serological markers of fibrosis
The APRI and FORNS were associated with fibrosis stage (P < 0.001), and their diagnostic performances are reported in Table 3 and 4. The AUROCs of APRI for fibrosis ≥F2 and cirrhosis were 0.805 (95% CI: 0.747-0.855) and 0.838 (95% CI: 0.783-0.884), respectively. Pairwise comparisons between APRI and FibroScan AUROCs showed that FibroScan® identified better both fibrosis ≥F2 (P < 0.001) and cirrhosis (P < 0.001). The AUROC of FORNS for fibrosis ≥F2 was 0.913 (95% CI: 0.867-0.947) comparable to FibroScan® (P = n.s.). Combination of APRI or FORNS with FibroScan® did not improve the performance of transient elastography alone (data not shown).
FibroTest and HA were measured in 164 consecutive patients, who were comparable to the overall population (Table 1), and both correlated with fibrosis stage (P < 0.001). The AUROCs of FT for fibrosis ≥F2 and cirrhosis were 0.892 (95% CI: 0.834-0.935) and 0.883 (0.824-0.928), respectively. The AUROCs of FibroScan® for both fibrosis ≥F2 and cirrhosis were better than those of FT (P =0.047 and P = 0.011, respectively, Fig. 4a,b). The diagnostic accuracy of FT for fibrosis ≥F2 was comparable to FibroScan® (Table 3). FT identified 52 of 90 cirrhotic patients (57.8% sensitivity), with 91.9% specificity and 89.7% PPV (Table 4).
The AUROC of HA for cirrhosis was 0.877 (95% CI: 0.817-0.923) (Fig. 4b). Pairwise comparisons between HA and FibroScan showed that FibroScan® performed better to detect cirrhosis (P = 0.006). Of 164 patients, 66 (40.2%) had HA levels >75 ng/mL and 58 of them were correctly classified as cirrhotics (64.4% sensitivity, 87.9% PPV) (Table 4).
Combination of FibroScan® with FT and/or HA did not improve the diagnostic performance of FibroScan alone, with the exclusion of cirrhotics with biochemical remission in whom the combination of FibroScan® with FT and HA showed higher sensitivity for diagnosis of cirrhosis: 80% for at least one test positive vs 40% for HA alone and 45% for FibroScan® or FT alone.
|
|
| |
| |
|
|
|