| |
Medicaids Restrictions Based on Misleading Information:
African-Americans/ Opoid Replacement (Methadone) Users SVR Rates, comorbidities
|
| |
| |
Jules Levin, NATAP
The medicaids that are imposing harsh & punitive restrictions to access for new HCV therapies are throwing up smokescreens to mislead the public, they claim the data/SVR rates are not good, studies haven't been conducted in drug users or African-Americans, but the real reason is they don't want to pay. In HIV they could never get away with this. Over $5 bill a year in spent on medicaid for HIV & $4.5 bill for medicare, and back when HAART first came out in 1996 they squawked as well, they didn't want to pay. The messaging by medicaid have been broadly distributed through the media & the public has eaten it up. But they have been sending out false & misleading information, particularly regarding the effectiveness of the SVR data from the new interferon-free regions, and regarding the effectiveness of the data in patient populations - African-Americans and opoid users; they are trying to deny therapy, they are scapegoating patients who are African-American & former substance abusers - those on opoid replacement therapy, (methadone). A key restriction to therapy access that some medicaids particularly Illinois recently constructed is related to patients with a history of substance abuse (including of note IDUs and alcohol!). Illinois guidelines recently released said anyone with evidence of substance abuse in the last 12 months could not get HCV therapy & they said there isn't data to say these patients respond as well as patients who don't have substance abuse concerns, the absurdity of this is that injection drug use is the leading cause of HHCV-infection so they want to deny access to the most significant patient group based on false information, see the data below - African-Americans, those in opoid replacement programs and HCV/HIV coinfected have the same response rates as everyone else. In fact the Illinois Medicaid Medical Director said in an interview 1 week ago: "one-third of the population for which we were approving Sovaldi take drugs or alcohol, and nobody ever studied if Sovaldi could be safe or effective for such people.......So we could not see everybody getting a prescription, just because their own data says it's effective about 90% of the time. The previously used drugs were effective 75% to 80% of the time. There's an edge for Sovaldi, but is it effective for population we serve? We can't answer that today. Many of our patients have co-morbidities and take other meds. We did not find evidence that Gilead did research on that type of population." ....http://www.natap.org/2014/HCV/080814_07.htm. This week Vertex announced they are stopping distribution of telaprevir in the USA. No one wants to take boceprevir or telaprevir+Peg/Rbv, the phase 3 studies in treatment-naive patients showed 70% SVR rates for these combinations which were 24-48 weeks of therapy and WITH interferon in phase 3 studies but the side effects were difficult to tolerate, with the new therapies in the same treatment-naive patient populations showing 95-100% SVR rates without interferon for 12 weeks therapy, and the side effects are much better, no interferon!. Oregon questioned all the data related to Sovaldi saying the data is just not proven to be adequately convincing yet! Medicaids have alluded to that Sovaldi has not been studied adequately or at all in African-Americans or as mentioned above in ovoid replacement patient populations, and of course they are alluding the same is true for all the new DAAs, week, this is not true......
AASLD/2013: Virologic Response Rates to Sofosbuvir-Containing Regimens Are Similar in Patients With and Without Traditional Negative Predictive Factors: a Retrospective Analysis of Phase 3 Data - (11/13/13)...these were studies of Sofosbuvir/Rbv but below are more recently presented data at EASL in April of the phase 3 Sofosbuvir/Ledipasvir ION studies
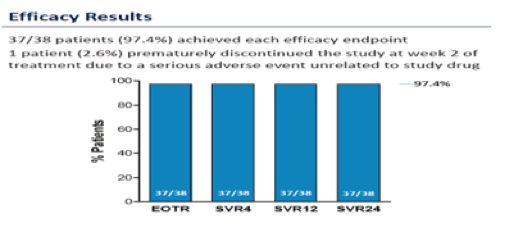
IAC: Abbvie 3D Regimen in HCV Genotype 1-Infected Patients on Methadone or Buprenorphine (07/21/14)
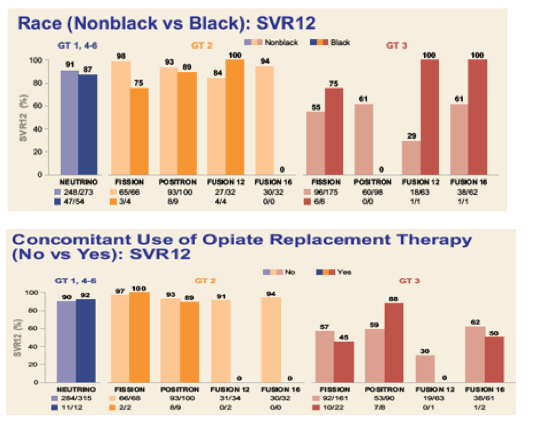
African-Americans
308 subjects (16%) in the ION studies were African American. Table 1 of each NEJM paper (attached) lists the demographic breakdown for the enrolled subjects.
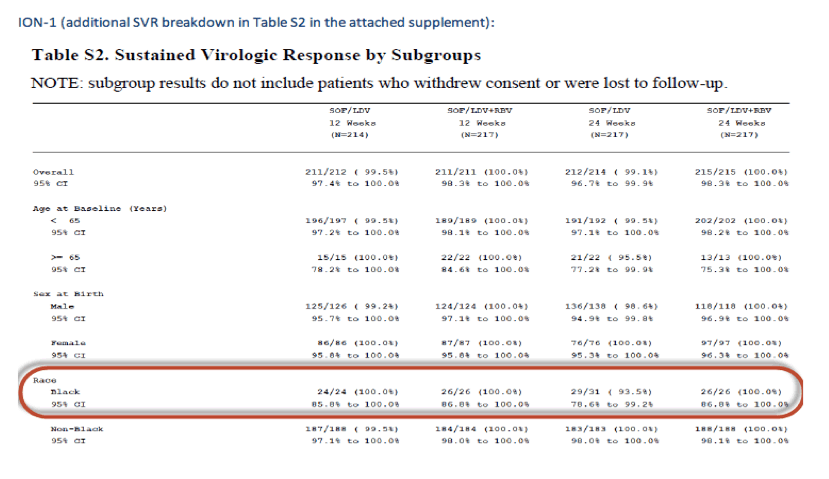
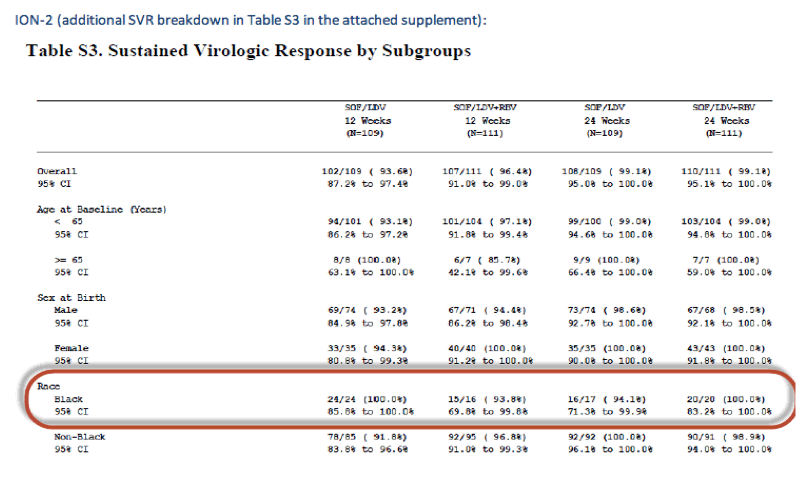
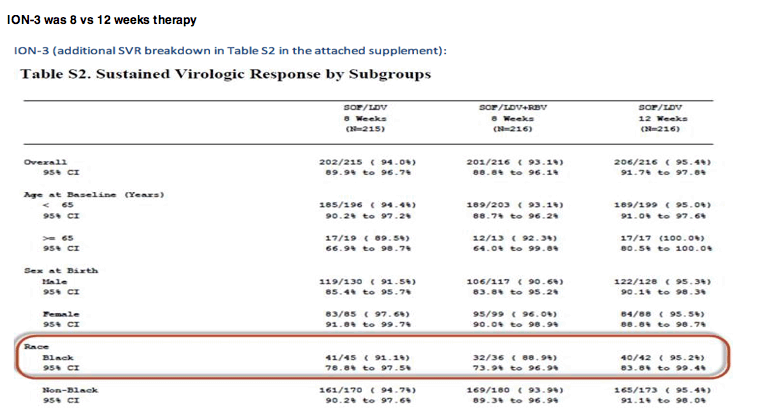
Below is the SVR breakdown for African American subjects (all public info from the ION NEJM supplements also attached).
IAC: SAPPHIRE-II Subgroup Analysis: ABT-450/r/Ombitasvir (ABT-267), Dasabuvir (ABT-333), and Ribavirin Regimen Achieves High Sustained Virologic Response Rates 12 Weeks Post-treatment in Treatment-experienced Patients With Chronic HCV GT 1 Infection, Regardless of Baseline Characteristics - (07/25/14)
IAC: SAPPHIRE-I Subpopulation Analysis: ABT-450/r/Ombitasvir (ABT-267), Dasabuvir (ABT-333), and Ribavirin Regimen Achieves High Sustained Virologic Response Rates 12 Weeks Post-treatment in Treatment-naïve Patients With Chronic HCV GT 1 Infection, Regardless of Baseline Characteristics - (07/25/14)
IAC: TURQUOISE-I: SAFETY AND EFFICACY OF ABT-450/R/OMBITASVIR, DASABUVIR, AND RIBAVIRIN IN PATIENTS CO-INFECTED WITH HEPATITIS C AND HIV-1 (07/21/14)
IAC: High SVR12 With Sofosbuvir/Ribavirin in HCV/HIV-Coinfected: PHOTON-2 (07/21/14)
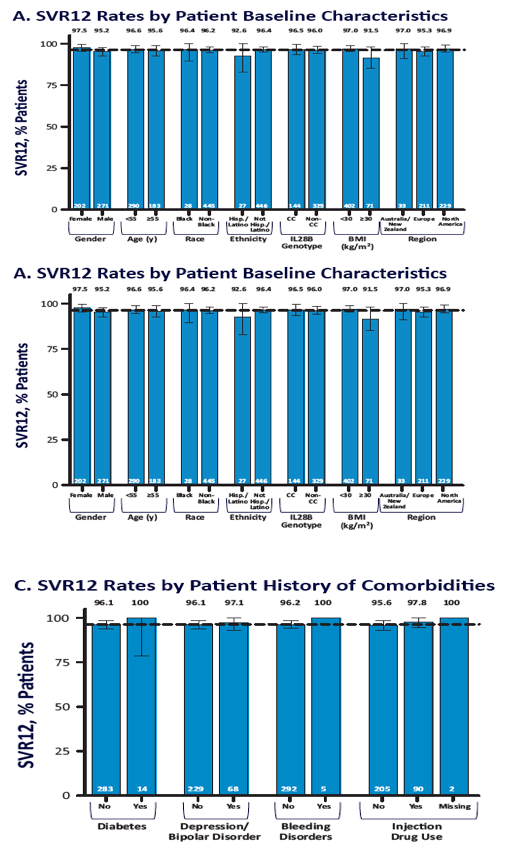
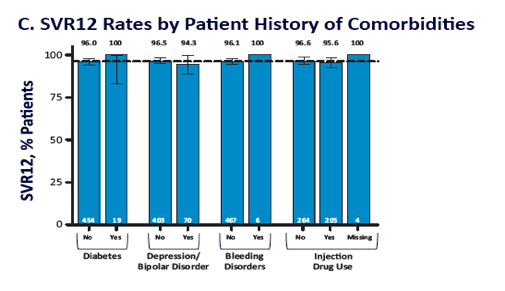
EASL: All Oral Fixed-Dose Combination Ledipasvir/Sofosbuvir With Or Without Ribavirin for 12 or 24 Weeks in Treatment-Naive Genotype 1 HCV-Infected Patients: the Phase 3 ION-1 Study - (04/14/14)
EASL: All Oral Fixed-Dose Combination Ledipasvir/Sofosbuvir With or Without Ribavirin for 12 or 24 Weeks in Treatment-Experienced Genotype 1 HCV-Infected Patients: The Phase 3 ION-2 Study - (04/14/14)
EASL: Ledipasvir/Sofosbuvir With and Without Ribavirin for 8 Weeks Compared to Ledipasvir/Sofosbuvir for 12 Weeks in Treatment-Naïve Noncirrhotic Genotype-1 HCV-Infected Patients: The Phase 3 ION-3 Study - (04/11/14)
NEJM publications, in the links you can find the full publications and the Supplementary Materials where the SVR rates by Race are provided, these tables above.
ION-1 Ledipasvir and Sofosbuvir for Untreated HCV Genotype 1 Infection - (04/14/14)
ION-2 Ledipasvir and Sofosbuvir for Previously Treated HCV Genotype 1 Infection - (04/14/14)
ION-3 Ledipasvir and Sofosbuvir for 8 or 12 Weeks for Chronic HCV without Cirrhosis - (04/11/14)
And here are the actual pdfs for the Supplementary Materials
Download the PDF here
Download the PDF here
Download the PDF here
EASL: TURQUOISE-II: SVR12 RATE OF 92-96% IN 380 HEPATITIS C VIRUS GENOTYPE 1-INFECTED ADULTS WITH COMPENSATED CIRRHOSIS TREATED WITH ABT-450/r/ABT-267 AND ABT-333 PLUS RIBAVIRIN - (04/14/14)
EASL: SAPPHIRE-I: PHASE 3 PLACEBO-CONTROLLED STUDY OF INTERFERON-FREE, 12-WEEK REGIMEN OF ABT-450/r/ABT-267, ABT-333, AND RIBAVIRIN IN 631 TREATMENT-NAïVE ADULTS WITH HEPATITIS C VIRUS GENOTYPE 1 - (04/11/14)
EASL: SAPPHIRE-II: PHASE 3 PLACEBO-CONTROLLED STUDY OF INTERFERON-FREE, 12-WEEK REGIMEN OF ABT-450/r/ABT-267, ABT-333, AND RIBAVIRIN IN 394 TREATMENT-EXPERIENCED ADULTS WITH HEPATITIS C VIRUS GENOTYPE 1 - (04/10/14)
TURQUOISE-II ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin for Hepatitis C with Cirrhosis - Phase 3 TURQUOISE-II - (04/14/14)
SAPPHIRE-I Treatment of HCV with ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin (in previously untreated patients with HCV genotype 1 infection and no cirrhosis) - (04/11/14)
SAPPHIRE-II Retreatment of HCV with ABT-450/r-Ombitasvir and Dasabuvir with Ribavirin - (04/10/14) NEJM
|
|
| |
| |
|
|
|