| |
'India Cries Out for HCV Services/Treatment' - Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study
|
| |
| |
Download the PDF here
The Lancet
Published online December 3, 2014
Sunil Suhas Solomon, Shruti H Mehta, Aylur K Srikrishnan, Suniti Solomon, Allison M McFall, Oliver Laeyendecker, David D Celentano,
Syed H Iqbal, Santhanam Anand, Canjeevaram K Vasudevan, Shanmugam Saravanan, Gregory M Lucas, Muniratnam S Kumar,
Mark S Sulkowski, Thomas C Quinn
HCV in India: low screening rates, poor awareness, genotype 3 prevalent: poor access to genotype testing, HCV quantification, harm reduction intervention needed/re-infection concern, alcohol use a problem- programs needed to address these
"HCV prevalence in HIV-infected people who inject drugs ranged from 18 (9·3%) of 197 to 213 (96·0%) of 226; seven of 15 sites had HCV prevalence of more than 75% in HIV-infected people. Unweighted estimates are listed in table 2."
"more than half of the study population in which more than one in three was infected with HCV had not heard of HCV. This poor knowledge translated into low testing rates and subsequently, very poor awareness of HCV status in those who were infected-the first step in the HCV care continuum. This poor knowledge coupled with low HCV treatment access is reminiscent of the HIV epidemic in people who inject drugs in India nearly 15 years ago. With HIV, only after treatment costs decreased substantially and programmes such as the Presidents Emergency Program for AIDS Relief (PEPFAR) and local governmental programmes improved access, was it recognised that most individuals were not accessing treatment because of insufficient awareness or engagement in care, prompting still ongoing efforts to improve outcomes along the continuum.29 Our data show that we face the same challenges with HCV and that although access to treatment might still be years away, literacy and testing programmes, especially in vulnerable populations should be implemented immediately."
"We have previously shown a preponderance of genotype 3 infection in people who inject drugs across India,28 similar to what is seen in other countries in Asia......guidelines call for HCV RNA and HCV genotyping to inform treatment decisions.26 In settings such as India, where access to HCV antibody testing itself is challenging as reported here, HCV genotyping and HCV RNA quantification are simply out of reach"
"In conclusion, these data clearly underline explosive epidemics of HCV in people who inject drugs in India with minimum access to HCV-related services and potentially representing scenarios in people who inject drugs in other resource-limited settings. Although there is heightened optimism about global eradication of HCV because of advances in therapeutics, these data underline several challenges, starting with low levels of awareness and access to HCV testing-the first steps in the pathway to global control of HCV infection. Although new treatments will become available in resource-limited settings in the near future, programmes to improve awareness and reduce disease progression and transmission need to be scaled up without further delay. Failure to do so will result in replication in resource-limited settings of mortality patterns reported in high-income settings and will also undermine the survival benefits of antiretroviral therapy noted in people infected with HIV who inject drugs."
"harm reduction interventions (eg, opioid substitution therapy and needle syringe exchange programmes) in highly vulnerable populations such as people who inject drugs will be crucial to minimise onward transmission of HCV.
Furthermore, when treatment does become available, harm reduction interventions will play a key part in minimising re-infection risk. This will be of crucial importance to cost-effectiveness of these novel drugs, whose pricing has already sparked much debate.30 In this study, rates of needle sharing were unacceptably high-eg, in Imphal where the HCV prevalence was 65%, needle sharing was reported by 80% of participants. Additionally, of the 79 persons who reported HCV treatment, only 26% were virus free at the time of the study, which probably shows some combination of non-response and re-infection, emphasising the potential benefits of harm-reduction interventions."
"Alcohol use is a well recognised driver of HCV disease progression.4 In this population, more than a third of people positive for HCV antibodies reported harmful or hazardous alcohol use. Interventions to reduce alcohol use in this population will help delay disease progression and, thereby, prolong survival until new antiviral agents are accessible. Moreover, although new antivirals are efficacious at clearing HCV infection even in cirrhotics, the management of cirrhosis itself will remain challenging in resource-limited settings, further emphasising the need for interventions to reduce alcohol use and halt progression to cirrhosis"
------------------------
Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study
The Lancet Infectious Diseases, Early Online Publication, 3 December 2014
Summary
Background
90% of individuals infected with hepatitis C virus (HCV) worldwide reside in resource-limited settings. We aimed to characterise the prevalence of HCV, HIV/HCV co-infection, and the HCV care continuum in people who inject drugs in India.
Methods
14 481 people (including 31 seeds-individuals selected as the starting point for sampling because they were well connected in the drug using community) who inject drugs were sampled from 15 cities throughout India using respondent-driven sampling from Jan 2, 2013 to Dec 19, 2013. Data from seeds were excluded from all analyses. HCV prevalence was estimated by the presence of anti-HCV antibodies incorporating respondent-driven sampling weights. HCV care continuum outcomes were self-reported except for viral clearance in treatment-experienced participants.
Findings
The median age of participants was 30 years (IQR 24-36) and 13 608 (92·4%) of 14 449 were men (data were missing for some variables). Weighted HCV prevalence was 5777 (37·2%) of 14 447; HIV/HCV co-infection prevalence was 2085 (13·2%) of 14 435. Correlates of HCV infection included high lifetime injection frequency, HIV positivity, and a high prevalence of people with HIV RNA (more than 1000 copies per mL) in the community. Of the 5777 people who inject drugs that were HCV antibody positive, 440 (5·5%) were aware of their status, 225 (3·0%) had seen a doctor for their HCV, 79 (1·4%) had taken HCV treatment, and 18 (0·4%) had undetectable HCV RNA. Of 12 128 participants who had not previously been tested for HCV, 6138 (50·5%) did not get tested because they had not heard of HCV. In the 5777 people who were HCV antibody positive, 2086 (34·4%) reported harmful or hazardous alcohol use, of whom 1082 (50·4%) were dependent, and 3821 (65·3%) reported needle sharing. Awareness of HCV positive status was significantly associated with higher education, HIV testing history, awareness of HIV positive status, and higher community antiretroviral therapy coverage.
Interpretation
The high burden of HCV and HIV/HCV co-infection coupled with low-access to HCV services emphasises an urgent need to include resource-limited settings in the global HCV agenda. Although new treatments will become available worldwide in the near future, programmes to improve awareness and reduce disease progression and transmission need to be scaled up without further delay. Failure to do so could result in patterns of rising mortality, undermining advances in survival attributed to widespread HIV treatment.
Funding
US National Institutes of Health
Introduction
About 184 million people are chronically infected with hepatitis C virus (HCV), of whom 90% reside in low-income and lower-middle income countries (countries with a gross national income per head of < US$4125).1, 2 People who inject drugs have a disproportionate prevalence (50-90%) of HCV infection worldwide compared with people who do not inject drugs.3 Chronic HCV infection is associated with substantial morbidity and mortality.4, 5 Unfortunately, most individuals infected with HCV are unaware of their infection because HCV is typically symptom free for decades.6 Treatment for chronic HCV is curative and substantial advances have occurred during the past few years.7-9 Short course interferon-free pan-genotypic regimens with cure rates of 95% are on the horizon.10 Subsequently, conversations about HCV eradication have begun.11, 12 However, such regimens will be expensive (similar to the early years of antiretroviral therapy [ART]) and access, particularly in hard-to-reach populations in resource-limited settings, will be the major challenge to the global control of HCV.13, 14
Little epidemiological and almost no data about access to HCV diagnostic and treatment services exist from resource-limited settings. Although barriers to HCV care have been well characterised in affluent settings,15 whether these will directly translate to resource-limited settings remains unknown.16
Epidemiological studies to understand disease burden and HCV service uptake are needed if global control of HCV infection is to become a reality.
The prevalence of HCV in the general population in India is about 1·0-1·9%.17 India has roughly 3 million opioid users, with as many as 1·1 million of these being injectors.18 We characterised the burden of HCV infection, HIV/HCV co-infection, the HCV care continuum, and associated factors in a large cross-sectional sample of drug users from 15 cities across India. This study was done as part of the baseline assessment of a cluster randomised trial. Stored specimens were tested for HCV antibodies.
Methods
Study design and participants
This study was done in 15 cities from 11 states in India (figure 1) as the baseline assessment of a cluster-randomised trial (NCT01686750). These cities were selected by study investigators and representatives of the National AIDS Control Organization (NACO), India, to represent regions with varying stages of drug use epidemics (established drug use epidemics, large cities, cities with documented emerging drug use epidemics, and cities with anecdotal evidence of emerging drug use epidemics) and different settings (large metropolitan cities, medium cities, and small cities). In each city, a local partner was identified that maintained a drop-in centre for people who inject drugs that provided some HIV prevention services (eg, opioid substitution). Only one study site was established in each city.
The eligibility criteria for inclusion in this study were being aged 18 years or older, providing a self-report of illicit drug injection in the previous 2 years, providing informed consent, and having a valid referral coupon. The study population was accrued using respondent-driven sampling, a chain-referral recruitment strategy that has been shown to be effective to recruit hard-to-reach populations, including people who inject drugs. Respondent-driven sampling uses systematically collected data about the relationships between recruiters and recruits, such that recruitment bias can be adjusted for in the analysis, resulting in estimates that are generalisable to the target population.19, 20 In each site, ethnography was done before the respondent-driven sampling with peer leaders (members of the drug-using community who mentors other members in the community) of people who inject drugs and key stakeholders in the local drug-using community to select so-called seeds (individuals who were identified during ethnography processes to be well connected in the drug using community). Recruitment of participants for this study was terminated early in the town of Moreh, India, because of civil unrest.
Procedures
After verbal consent, participants provided a fingerprint image to avoid duplicate enrolment. Participants completed an interviewer-administered survey, which captured information on demographics, the network characteristics of people who inject drugs (relationship to recruiter and network size), risk behaviour, HIV and HCV testing, and treatment and substance use, including alcohol (using the Alcohol Use Disorders Identification Test [AUDIT]).
Participants underwent rapid HIV testing with pre-test and post-test counselling. Samples collected for future testing were shipped to the central laboratory in Chennai. Seeds and participants were given two hologram-labelled referral coupons to give to any two members of their network. All participants received a monetary incentive of 50 Indian rupees (INR); (about US$0·8) for each participant referred who completed study procedures.
HIV infection was diagnosed on-site using three rapid tests: Alere Determine and HIV-1/2 test (Alere Medical Company, Chiba, Japan), the first response HIV card test 1-2·0 (PMC Medical India Pvt, Daman, India), and Signal Flow Through HIV 1 + 2 Spot/Immunodot Test kit, (Span Diagnostics, Surat, India). HIV-1 RNA was quantified from fresh plasma specimen using the RealTime HIV-1 assay (Abbott Laboratories, Abbott Park, IL, USA). HCV antibody testing was done on stored specimens using the Genedia HCV ELISA 3.0 (Green Cross Medical Science, Chungbuk, Korea). HCV RNA was quantified in stored specimens from participants who reported receiving HCV treatment in the baseline survey (n=79) using the RealTime HCV assay (Abbott Molecular, Des Plaines, IL, USA); HCV RNA levels with less than 30 copies per mL were judged to be representative of a sustained virological response.
Outcomes
The primary outcome was HCV prevalence, which was measured by detection of HCV antibodies. Secondary outcomes were HCV care continuum outcomes, including linkage to care, initiation of HCV treatment, and sustained virological response. Linkage to care and initiation of HCV treatment were ascertained by self-report. People were considered to be linked to care if they reported ever seeing a doctor, nurse, or other health-care professional for their hepatitis C infection management. People were considered to have initiated HCV treatment if they reported ever receiving interferon, pegylated interferon or ribavirin, or both. Sustained virological response was ascertained by testing for HCV RNA in all people who reported taking HCV treatment on one or more occasions in the past.
Statistical analysis
Data from seeds were excluded from analyses. The respondent-driven sampling II estimator (Volz-Heckathorn), which weights for network effects, was used to calculate site-specific estimates.21 All percentages reported in the text incorporate these weights (unweighted estimates are in the appendix). Weights incorporate self-reported network size (number of people who inject drugs seen in the previous 30 days). Also, a composite weight, which accounts for the number of people who inject drugs in each city derived from state-level data22 was used to estimate overall proportions. Several assumptions are important when using samples obtained from respondent-driven sampling to generalise to the target population, such as depth of recruitment (greater than six waves of recruitment), homophily (tendency to recruit others with similar characteristics), and equilibrium (sample characteristics are independent of seeds).19 Our respondent-driven sampling achieved a median depth of 22 waves (IQR 12-50) across the 15 sites. Overall, homophily values for HCV status were low (IQR -0·031 to 0·134 in people not infected with HCV; IQR 0·002-0·444 in people infected with HCV). All samples achieved equilibrium. The appendix provides site-wise respondent driven sampling process measures.
Individual-level and site-level correlates of HCV antibody positivity were identified using multi-level logistic regression with random-intercepts per site (to account for clustering) incorporating scaled respondent driven sampling II weights. Individual correlates included demographics and lifetime risk behaviours. Site-level variables included median network size (self-reported by people who inject drugs) and prevalence of HIV viremia (number of people in a community who had more than 1000 copies per mL of HIV RNA); we deemed this a measure of HIV prevalence and service access in the city. All variables significantly associated with HCV prevalence in univariable analysis (p<0·05) and some variables deemed important a priori (age, sex, education, income, and region) were selected for inclusion in the multivariable model. With the exception of age, which was included irrespective of statistical significance, only those variables associated with the outcome at p<0·05 were retained in the final multivariable model.
HCV care continuum outcomes were based on self-reported data with the exception of a sustained virological response, which was based on HCV RNA testing of specimens from 79 participants who reported receiving HCV treatment on one or more occasion. Participants were asked if they had ever been to a physician to discuss their HCV to establish their linkage to care.
Correlates of awareness of HCV positive status were estimated using methods similar to the HCV prevalence analysis, multi-level logistic regression accounting for clustering by site incorporating respondent driven sampling II weights. Antiretroviral therapy coverage in the community was selected as an additional site-level variable in this analysis as a measure of access to health services in the city. Because of strong co-linearity between region and the site-level correlates for network size and the proportion of people with HIV receiving antiretroviral therapy, two different multivariable models were constructed. Model 1 includes the region correlate, whereas model 2 includes the site-level correlates for network size and proportion of people with HIV receiving ART in addition to individual-level correlates. The number of years of injection drug use is not included in the final multivariate models because of its co-linearity with age. All statistical analyses were done using respondent driven sampling Analyst Software Version 0·1 and STATA Version 12·0 (College Station, Texas, US).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the Article. SSS had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The median age of the 14 450 people who inject drugs was 30 years (IQR 24-36). Most of the drug users in this study were men (13 608 [92·4%] of 14 449 [data were missing for some variables]), 5882 (40·7%) were married, and 5018 (39·2%) had primary school education or less. The median monthly income was US$82 (IQR 33-115) and the median age at first injection was 21 years (IQR 18-26). Overall, 5815 (47·7%) of the participants reported sharing injection paraphernalia on one or more occasions and 5838 (42·2%) had evidence of harmful or hazardous alcohol use or dependence. HIV prevalence was 2905 (21·1%).
Substantial variability was seen across sites (table 1). Median age ranged from 24 to 34 years and the proportion of women from one (0·1%) of 1000 to 200 (23·3%) of 1000. The age at first injection ranged from 18 to 26 years. Harmful or hazardous alcohol use ranged from 69 (5·5%) of 999 to 297 (34·5%) of 1000 and alcohol dependence ranged from 33 (6·0%) of 457 to 524 (51·0%) of 1000. The proportion of people who reported needle sharing on one or more occasions ranged from 261 (19·9%) of 1000 to 804 (80·4%) of 1000. HIV prevalence ranged from 61 (5·9%) of 1000 in Bhubaneswar to 198 (44·9%) of 457 in Moreh. For unweighted estimates, see appendix.
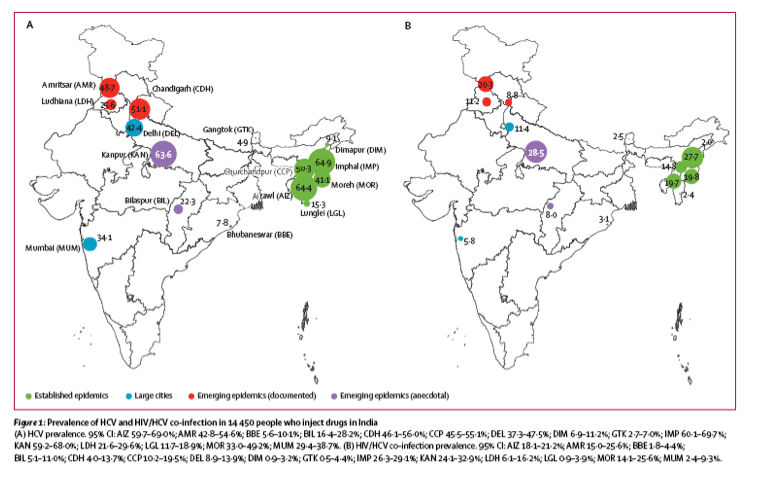
Overall weighted HCV prevalence was 5777 (37·2%) of 14 447 (95% CI 36·3-38·0). Site-specific weighted HCV prevalence ranged from 41 (4·9%) of 1000 in Gangtok to 684 (64·9%) of 1000 in Imphal (figure 1). Weighted HIV/HCV co-infection prevalence was 2085 (13·2%) of 14 435 (95% CI 12·7-13·7) (figure 1). Site-specific weighted HIV/HCV co-infection prevalence ranged from 18 (2·0%) of 996 in Dimapur to 323 (28·5%) of 999 in Kanpur (figure 1). HCV prevalence in HIV-infected people who inject drugs ranged from 18 (9·3%) of 197 to 213 (96·0%) of 226; seven of 15 sites had HCV prevalence of more than 75% in HIV-infected people. Unweighted estimates are listed in table 2.
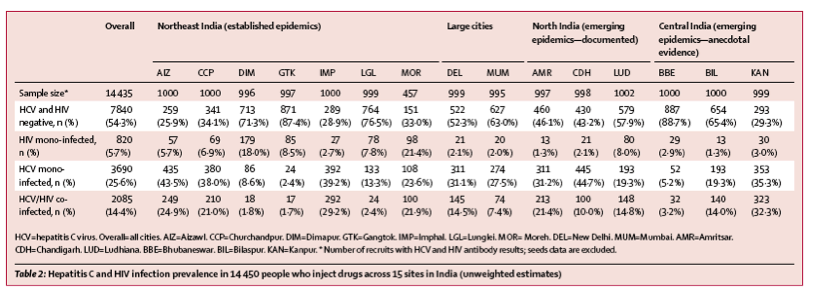
Table 3 lists the unadjusted and adjusted weighted correlates of HCV infection. In multivariable analysis, male sex, ever visiting a needle exchange programme, ever visiting an opioid substitution therapy (OST) centre, higher number of lifetime injections, younger age at first injection, injecting drugs in combination and a history of sharing needles were significantly associated with HCV infection. HIV-infected drug users were more likely to be infected with HCV (OR 5·25, 95% CI 3·41-8·09). Drug users from cities with higher HIV viremia had higher HCV prevalence (OR for site-level HIV viremia >18% compared with ²6%, 6·82, 95% CI 2·27-20·5) similar to drug users in cities with larger networks of people who inject drugs (OR for site-level median network size of >25 injectors seen in previous 30 days compared with site-level median of <12 injectors is 3·45, 95% CI 2·0-5·96). Inferences were unchanged in sensitivity analyses that were unweighted (appendix) or used RDS-I weights23 (data not shown).
1272 (7·0% weighted) of 14 450 people in this study had been tested for HCV on one or more occasion. The most common reason for not getting tested was not having heard of HCV (6138 [50·5%] of 12 128). Of those who had heard of HCV, low perceived risk (4374 [73·2%] of 6047) and not knowing where to get tested (937 [14·3%] of 6047) were common reasons for not getting tested. Of those tested, 717 (55·3%) of 1272 were tested in private and 491 (41%) of 1272 in government centres; 580 (52·6%) of 1272 reported being tested because they wanted to learn their status and 307 (24·9%) of 1272 were referred by a physician. HCV prevalence in people tested was 869 (61·6%) of 1272 compared with 4635 (35·3%) of 12 340 in those not tested. In people positive for the HCV antibody, 2086 (34·4%) of 5777 reported harmful or hazardous alcohol use; of whom 1082 (50·4%) of 2086 were alcohol dependent.
Of 5777 drug users positive for the HCV antibody, 440 (5·5%) were aware of their status, 225 (3·0%) had been to see a doctor for their HCV (linked to care), 79 (1·4%) had taken HCV treatment, and 18 (0·4%) had undetectable HCV RNA at the time of the survey (26·4% of those who reported taking treatment; figure 2). Cities with established epidemics had marginally better outcomes along the HCV care continuum than cities without established epidemics; cities with anecdotal evidence of emerging drug use fared worst (appendix).
Table 4 lists unadjusted and adjusted weighted correlates of HCV-positive awareness and the appendix lists unweighted estimates. In multivariable analysis (model 1), awareness of HCV positivity was significantly associated with age (OR per 10 year increase 1·55, 95% CI 1·27-1·90), higher education (OR for high school vs primary school 3·75, 2·10-6·72), receiving opioid substitution therapy on one or more occasion (OR 1·87, 95% CI 1·05-3·32), and being tested for HIV (OR 3·52, 95% CI 2·35-5·26). Compared with people who were HIV negative, those who were HIV positive and were aware of their status were more likely to also be aware of their HCV infection (OR 3·83, 95% CI 1·85-7·95); being HIV-positive but unaware of status did not increase likelihood of knowledge of HCV status. People from regions other than those with established epidemics were significantly less likely to be aware of their HCV-positive status (OR for emerging epidemics [anecdotal] 0·11, 95% CI 0·05-0·25). In models with site-level correlates (model 2), drug users from cities with better antiretroviral therapy coverage and larger drug-using networks were more likely to be aware of their HCV positive status.
79 participants reported receiving some form of interferon-based treatment for their HCV; 61 (77·2%) were from cities with established epidemics. 17 (21·6%) reported discontinuing treatment. 56 (70·8%) received treatment in a private setting. Compared with drug users who were not treated, those treated were significantly more likely to be educated, to have been tested for HIV and if positive, on antiretroviral therapy (p<0·01 for all).
Discussion
This study shows a high burden of HCV and HIV/HCV co-infection in one of the largest samples of people who inject drugs from a resource-limited setting (panel). Despite this high burden, fewer than half of the participants had heard of hepatitis C, and of those who were infected with HCV, only one in 20 were aware of their status. By contrast, in the USA, 50% of people infected with HCV are aware of their infection.6 Although the HCV care continuum has been described as a cascade in such high-income regions, these data suggest that in this population of people who inject drugs in India, the care continuum is more suggestive of a cliff. In this time of rapidly expanding HCV therapeutic optimism, these data have important implications for global HCV control.
--------------
Panel
Research in context
Systematic Review
We did two independent PubMed searches on Aug 27, 2014, corresponding to the two major objectives of our paper: epidemiology (prevalence of HCV and HIV/HCV co-infection) and the HCV care continuum. We used terms for hepatitis C (eg, "hepatitis C" and "HCV"), "prevalence" or "epidemiology", people who inject drugs (eg, "IDU" and "people who inject drugs"), and low-middle income countries (eg, "less developed countries" and "India") for the epidemiology search and identified 121 publications. We used the same terms for hepatitis C and people who inject drugs with additional terms for care continuum (eg, "linkage to care" and "continuity of patient care") for the care continuum search and identified 143 publications. We identified reports of epidemiological studies of prevalence of HCV and HIV/HCV co-infection in people who inject drugs, review articles on the prevalence of HCV in people who inject drugs in low-income and middle-income countries and reports of the management of HCV infection in people who inject drugs. Of the 143 publications related to the care continuum, we did not identify any studies that reported the HCV care continuum in people who inject drugs from any low-income and middle-income countries. There is an increased sense of optimism about HCV management in view of advances in HCV therapeutics. In fact, editorials have cited the "beginning of the end" of the HCV epidemic. In April, 2014, WHO released their first ever guidelines for the management of HCV infection. In these guidelines, they recognised a gap in the knowledge of epidemiology and management of HCV in persons living in low-income and middle-income countries because most data related to the management of HCV and the HCV care continuum came from high-income settings. Review articles in the past have shown that there is a high burden of HCV infection in people who inject drugs worldwide, with people who inject drugs in low-income and middle-income countries bearing a higher burden. Beyond data about prevalence, little information exists about challenges that one might face in the global control of HCV infection.
Interpretation
Our study identified at least three important findings that are crucial to the global HCV control agenda. First, to achieve global control of HCV infection, hard-to-treat populations such as people who inject drugs in low-income and middle-income countries need to be targeted as they bear a substantial burden of HCV and HIV/HCV infection. This study, one of the largest epidemiological studies in people who inject drugs in a low to middle income country, identified that about one in three people who inject drugs was infected with HCV and more than one in ten were co-infected with HIV/HCV. Failure to address HCV will result in increased mortality because of HCV in low-income and middle-income countries, especially in HIV/HCV co-infected people who inject drugs-a trend reported in high-income settings. Second, although most of the debate surrounding management of HCV in low-income and middle-income countries has been focused on cost of the novel drugs, our data clearly show that there are several steps before treatment that need to be addressed. Less than 50% of people who inject drugs had heard of HCV and only about one in 20 people who inject drugs infected with HCV were aware of their HCV positive status. Until treatment becomes available, interventions need to focus on improvement of HCV literacy and improvement of access to HCV testing-both antibody and HCV RNA, which is essential to establish eligibility for HCV therapy. Third, there is a high prevalence of risk behaviours that could jeopardise the effect of the treatment. High rates of needle sharing as noted in our study could result in high rates of re-infection, adversely affecting the cost-effectiveness of these novel regimens and high levels of alcohol consumption could result in faster progression to liver cirrhosis-cirrhotics have been shown to respond to therapy less favourably than non-cirrhotics. Therefore, before widespread rollout of HCV therapy, it is essential to scale up harm reduction interventions and introduce programmes to reduce alcohol use in these populations to maximise effectiveness of HCV therapy.
--------------------
As expected, HCV prevalence in people who inject drugs in India was high: 1·5 to 2 times the HIV prevalence in most sites, showing the high transmissibility and large reservoir of HCV in people who inject drugs.24 However, significant variability was noted across sites probably because of diversity in drug use epidemic stage and sociodemographic and risk behaviours. For the most part, correlates of HCV infection were as expected. We did note a positive association between attending a syringe and needle exchange programme (SNEP) or OST programme and HCV prevalence, which likely shows that the heaviest injectors and potentially those with the highest levels of risk behaviour tend to be referred for these services. Interestingly, community HIV viraemia, a surrogate for access to HIV prevention and treatment services,25 was strongly associated with HCV prevalence, suggesting the need to integrate HCV-related services into existing HIV programmes for people who inject drugs, a strategy endorsed by WHO.26 Integration is further supported by the high co-infection prevalence reported in this study and the strong association between awareness of HIV positive status and awareness of HCV positive status. The Indian national programme is focused on improving HIV-related service access for people who inject drugs. Although this will decrease AIDS-related mortality, India might begin to notice increased HCV-related mortality if access to HCV services remains unchanged, similar to what is being seen in high-income settings.27 In a previous study, we identified that liver-related mortality is already a serious concern in northeast India, where drug use has been endemic for decades and people who inject drugs have above average access to antiretroviral therapy compared with people who inject drugs in other regions of India.28
In April, 2014, WHO published its first ever guidelines on HCV management showing a changing global perspective towards HCV.26 They recommended that all persons with chronic HCV, including people who inject drugs, be assessed for treatment, with prioritisation of patients with advanced fibrosis or cirrhosis. The guidelines call for epidemiological data in resource-limited settings. Several of the findings from this study will inform successful implementation of these guidelines.
First, more than half of the study population in which more than one in three was infected with HCV had not heard of HCV. This poor knowledge translated into low testing rates and subsequently, very poor awareness of HCV status in those who were infected-the first step in the HCV care continuum. This poor knowledge coupled with low HCV treatment access is reminiscent of the HIV epidemic in people who inject drugs in India nearly 15 years ago. With HIV, only after treatment costs decreased substantially and programmes such as the Presidents Emergency Program for AIDS Relief (PEPFAR) and local governmental programmes improved access, was it recognised that most individuals were not accessing treatment because of insufficient awareness or engagement in care, prompting still ongoing efforts to improve outcomes along the continuum.29 Our data show that we face the same challenges with HCV and that although access to treatment might still be years away, literacy and testing programmes, especially in vulnerable populations should be implemented immediately.
Second, as outlined by WHO, management of HCV infection is about more than antiviral drugs. Alcohol use is a well recognised driver of HCV disease progression.4 In this population, more than a third of people positive for HCV antibodies reported harmful or hazardous alcohol use. Interventions to reduce alcohol use in this population will help delay disease progression and, thereby, prolong survival until new antiviral agents are accessible. Moreover, although new antivirals are efficacious at clearing HCV infection even in cirrhotics, the management of cirrhosis itself will remain challenging in resource-limited settings, further emphasising the need for interventions to reduce alcohol use and halt progression to cirrhosis.
Third, harm reduction interventions (eg, opioid substitution therapy and needle syringe exchange programmes) in highly vulnerable populations such as people who inject drugs will be crucial to minimise onward transmission of HCV. Furthermore, when treatment does become available, harm reduction interventions will play a key part in minimising re-infection risk. This will be of crucial importance to cost-effectiveness of these novel drugs, whose pricing has already sparked much debate.30 In this study, rates of needle sharing were unacceptably high-eg, in Imphal where the HCV prevalence was 65%, needle sharing was reported by 80% of participants. Additionally, of the 79 persons who reported HCV treatment, only 26% were virus free at the time of the study, which probably shows some combination of non-response and re-infection, emphasising the potential benefits of harm-reduction interventions.
Finally, HCV therapeutics are rapidly evolving. Simeprevir and sofosbuvir, recommended for use in current guidelines, have shown a higher efficacy in genotype 1 infection versus genotype 2 or 3.26, 31 We have previously shown a preponderance of genotype 3 infection in people who inject drugs across India,28 similar to what is seen in other countries in Asia. Moreover, guidelines call for HCV RNA and HCV genotyping to inform treatment decisions.26 In settings such as India, where access to HCV antibody testing itself is challenging as reported here, HCV genotyping and HCV RNA quantification are simply out of reach. For comparison, despite the widespread global availability of ART since the early 2000s, HIV RNA quantification is seldom done in resource-limited settings because of inadequate infrastructure and cost. Even if new pan-genotypic regimens become available, HCV RNA testing will remain a crucial part of HCV management, making low-cost methods for identification of HCV RNA essential. Furthermore, in resource-limited settings, continuing medical education is not common, which can be challenging in view of the rapid pace at which HCV therapeutics are evolving. Currently, only pegylated interferon and ribavirin are available in India, but the cost of a 24 week regimen of pegylated interferon and ribavirin is INR 140 000-about three to five times the annual income of people who inject drugs recruited in this study. Moreover, patients need to pay for HCV treatment out-of-pocket making treatment unaffordable for most of people who inject drugs. However, although treatment cost is and will remain a major barrier to management of HCV infection in resource-limited settings, these data collectively underline several other challenges that need to be addressed alongside improvement of treatment access for global HCV control.
Our analysis had several limitations. All outcomes along the care continuum except for viral clearance in those who received treatment were ascertained via self-report and, therefore, bear limitations related to recall bias. The absence of HCV RNA data on all samples precludes our ability to estimate the proportion actually needing treatment and the proportion that were virus-free. However, if we recalibrated estimates to account for clearance previously reported in people who inject drugs in India (about 30%),32 the proportion needing treatment that received treatment would still be 2%. Respondent driven sampling needs several assumptions to arrive at a truly representative sample, only some of which can be validated (eg, depth of recruitment, homophily). A key assumption in estimation of respondent driven sampling weights that cannot be validated is the participants' network size because this information was obtained by self-report from the participant; however, we do not foresee any reason for participants to misreport their network size.
In conclusion, these data clearly underline explosive epidemics of HCV in people who inject drugs in India with minimum access to HCV-related services and potentially representing scenarios in people who inject drugs in other resource-limited settings. Although there is heightened optimism about global eradication of HCV because of advances in therapeutics, these data underline several challenges, starting with low levels of awareness and access to HCV testing-the first steps in the pathway to global control of HCV infection. Although new treatments will become available in resource-limited settings in the near future, programmes to improve awareness and reduce disease progression and transmission need to be scaled up without further delay. Failure to do so will result in replication in resource-limited settings of mortality patterns reported in high-income settings and will also undermine the survival benefits of antiretroviral therapy noted in people infected with HIV who inject drugs.
|
|
| |
| |
|
|
|