| |
HIV-Infected Men at Increased Risk for Heart Disease, Large NIH Study Finds
|
| |
| |
Download PDF here
(full-text below)
NIH-Supported Research Also Identifies Predictors of Heart Disease Risk In This Group
The buildup of soft plaque in arteries that nourish the heart is more common and extensive in HIV-infected men than HIV-uninfected men, independent of established cardiovascular disease risk factors, according to a new study by National Institutes of Health grantees. The findings suggest that HIV-infected men are at greater risk for a heart attack than their HIV-uninfected peers, the researchers write in Annals of Internal Medicine.
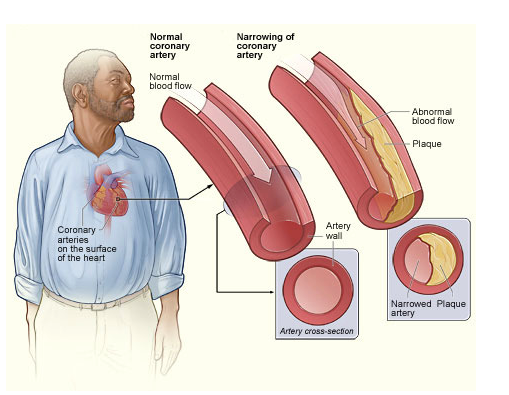
Plaque buildup in the arteries that nourish the heart, a condition called coronary atherosclerosis, narrows the arteries and increases the risk for heart attack.
Credit: NHLBI
In addition, blockage in a coronary artery was most common among HIV-infected men whose immune health had declined the most over the course of their infection and who had taken anti-HIV drugs the longest, the scientists found, placing these men at even higher risk for a heart attack.
"These findings from the largest study of its kind tell us that men with HIV infection are at increased risk for the development of coronary artery disease and should discuss with a care provider the potential need for cardiovascular risk factor screening and appropriate risk reduction strategies," said Gary H. Gibbons, M.D., director of the National Heart, Lung, and Blood Institute (NHLBI), part of NIH.
"Thanks to effective treatments, many people with HIV infection are living into their 50s and well beyond and are dying of non-AIDS-related causes--frequently, heart disease," said Anthony S. Fauci, M.D., director of the National Institute of Allergy and Infectious Diseases (NIAID), also part of NIH. "Consequently, the prevention and treatment of non-infectious chronic diseases in people with HIV infection has become an increasingly important focus of our research."
NIAID and NHLBI funded the study with additional support from the National Center for Advancing Translational Sciences, part of NIH.
Past studies of the association between heart disease and HIV infection have reached inconsistent conclusions. To help clarify whether an association exists, the current investigation drew participants from the Multicenter AIDS Cohort Study (MACS), a study of HIV/AIDS in gay and bisexual men established by NIAID nearly 30 years ago.
"One advantage of the MACS is that it includes HIV-uninfected men who are similar to the HIV-infected men in the study in their sexual orientation, lifestyle, socioeconomic status and risk behavior, which makes for a good comparison group," said Wendy S. Post, M.D., who led the study. Dr. Post is a professor of medicine and epidemiology at the Johns Hopkins School of Medicine and the Johns Hopkins Bloomberg School of Public Health in Baltimore.
Another advantage was the MACS' size, with nearly 7,000 men cumulatively enrolled, 1,001 of whom participated in the new study. The participants included 618 men who were HIV-infected and 383 who were not. All were 40 to 70 years of age, weighed less than 200 pounds, and had had no prior surgery to restore blood flow to the coronary arteries.
Dr. Post and colleagues investigated whether the prevalence and extent of plaque buildup in coronary arteries, a condition called coronary atherosclerosis, is greater in HIV-infected men than HIV-uninfected men and whether that plaque is soft or hard. Coronary atherosclerosis, especially soft plaque, is more likely to be a precursor of heart attack than hard plaque.
The scientists found coronary atherosclerosis due to soft plaque in 63 percent of the HIV-infected men and 53 percent of the HIV-uninfected men. After adjusting for cardiovascular disease risk factors, including high blood pressure, diabetes, high cholesterol, high body mass index and smoking, the presence of soft plaque and the cumulative size of individual soft plaques were significantly greater in men with HIV infection.
In addition, by examining a subgroup of HIV-infected men, the scientists discovered two predictors of advanced atherosclerosis in this population. The first predictor deals with white blood cells called CD4+ T cells, which are the primary target of HIV and whose level, or count, is a measure of immune health. The researchers found that for every 100 cells per cubic millimeter decrease in a man's lowest CD4+ T cell count, his risk of coronary artery blockage rose by 20 percent. The scientists also found that for every year a man had taken anti-HIV drugs, his risk of coronary artery blockage rose by 9 percent.
Because the investigators examined coronary artery plaque at a single point in time, further research is needed to determine whether coronary artery plaque in HIV-infected men is less likely to harden over time, or whether these men simply develop greater amounts of soft plaque, according to Dr. Post. In addition, she said, studies on therapies and behavioral changes to reduce risk for cardiovascular disease in men and women infected with HIV are needed to determine how best to prevent progression of atherosclerosis in this population.
----------------------------------------
Ann Intern Med 1 April 2014
"In conclusion, noncalcified coronary artery plaque was more prevalent and extensive in HIV-infected men, suggesting increased risk for cardiovascular events. Men with more advanced HIV infection, which is shown by low nadir CD4+ T-cell count and a greater number of years receiving HAART, have a higher prevalence of clinically significant coronary stenosis greater than 50%.
Additional studies are needed to identify how best to prevent progression of atherosclerosis in this unique population and the correlation of atherosclerosis with future events. Although coronary CT angiography is not indicated as a screening test in asymptomatic persons, these results emphasize the importance of assessing and modifying traditional cardiovascular risk factors in this population, especially in men with a history of low nadir CD4+ T-cell count."
"In MACS, we found a greater prevalence and extent of coronary atherosclerosis on coronary CT angiography among HIV-infected men than among uninfected men. Coronary CT angiography allows for identification of specific plaque composition. Noncalcified plaque prevalence and extent were each independently and positively associated with HIV-positive serostatus even after adjustment for CAD risk factors. Noncalcified plaques may be more prone to rupture, leading to acute coronary syndromes (15-16). However, in this cohort, relatively few coronary clinical events have been identified to date. Among HIV-infected men, coronary artery stenosis greater than 50% was associated with lower nadir CD4+ T-cell count and longer treatment with HAART."
"Of note, MACS includes a control group of uninfected men drawn from the same reference population with a similar lifestyle-men who have sex with men-and this helps to minimize unmeasured potential confounding and selection bias.A smaller study of 102 HIV-infected men and women and 41 uninfected control participants also found more coronary segments containing non calcified plaque among HIV-infected patients (24).
The prevalence of noncalcified plaque increased with advancing age in HIV-infected men but not in uninfected men, which is also consistent with the higher prevalence in infected men that is seen as early as age 50 years (Figure). Additional studies that include repeated longitudinal assessments of plaque in this cohort are needed to determine whether noncalcified plaques calcify at a slower rate in HIV-infected men or whether the incidence of new non calcified plaques is increased.
We found positive associations between the presence of coronary artery stenosis greater than 50% and both a lower nadir CD4 T-cell count and greater years of HAART. Low CD4 T-cell count has been associated with carotid intima-media thickness (10) and carotid artery plaque (25), although these associations were not seen in some other studies (26). The SMART study showed
that continuous use of antiretroviral therapy reduced CAD events compared with interrupted antiretroviral therapy (8). Coronary artery stenosis greater than 50% is an indication of advanced atherosclerosis. Lower nadir CD4 T-cell count is probably a marker of longer duration of uncontrolled viremia in years before the initiation of effective antiretroviral therapy. In addition, lower nadir CD4 T-cell count and longer duration of HAART are markers of longer duration of HIV infection and exposure to older formulations of antiretroviral therapy, with greater potential adverse metabolic effects, although controlling for traditional CAD risk factors accounts for some of these effects. Our future studies will investigate associations between coronary atherosclerosis with specific antiretroviral therapy regimens and measures of inflammation and immune activation."
Associations Between HIV Infection and Subclinical Coronary Atherosclerosis
Wendy S. Post, MD, MS; Matthew Budoff, MD; Lawrence Kingsley, PhD; Frank J. Palella Jr., MD; Mallory D. Witt, MD; Xiuhong Li, MS; Richard T. George, MD; Todd T. Brown, MD, PhD; and Lisa P. Jacobson, ScD
Abstract
Background: Coronary artery disease (CAD) has been associated with HIV infection, but data are not consistent.
Objective: To determine whether HIV-infected men have more coronary atherosclerosis than uninfected men.
Design: Cross-sectional study.
Setting: Multicenter AIDS Cohort Study.
Participants: HIV-infected (n = 618) and uninfected (n = 383) men who have sex with men who were aged 40 to 70 years, weighed less than 136 kg (200 lb), and had no history of coronary revascularization.
Measurements: Presence and extent of coronary artery calcium (CAC) on noncontrast cardiac computed tomography (CT) and of any plaque; noncalcified, mixed, or calcified plaque; or stenosis on coronary CT angiography.
Results: 1001 men had noncontrast CT, of whom 759 had coronary CT angiography.
After adjustment for age, race, CT scanning center, and cohort, HIV-infected men had a greater prevalence of CAC (prevalence ratio [PR], 1.21 [95% CI, 1.08 to 1.35]; P = 0.001) and any plaque (PR, 1.14 [CI, 1.05 to 1.24]; P = 0.001), including noncalcified (PR, 1.28 [CI, 1.13 to 1.45]; P < 0.001) and mixed (PR, 1.35 [CI, 1.10 to 1.65]; P = 0.004) plaque, than uninfected men. Associations between HIV infection and any plaque or noncalcified plaque remained significant (P < 0.005) after CAD risk factor adjustment. HIV-infected men had a greater extent of noncalcified plaque after CAD risk factor adjustment (P = 0.026). They also had a greater prevalence of coronary artery stenosis greater than 50% (PR, 1.48 [CI, 1.06 to 2.07]; P = 0.020), but not after CAD risk factor adjustment. Longer duration of highly active antiretroviral therapy (PR, 1.09 [CI, 1.02 to 1.17]; P = 0.007) and lower nadir CD4+ T-cell count (PR, 0.80 [CI, 0.69 to 0.94]; P = 0.005) were associated with coronary stenosis greater than 50%.
Limitation: Cross-sectional observational study design and inclusion of only men.
Conclusion: Coronary artery plaque, especially noncalcified plaque, is more prevalent and extensive in HIV-infected men, independent of CAD risk factors.
----------------------------
Editors' Notes
Context
· Data conflict about whether HIV infection or its treatment may increase a person's risk for chronic diseases, such as coronary artery disease.
Contribution
· HIV-infected and uninfected men had cardiac computed tomography. After adjustment for demographic variables and known risk factors for coronary artery disease, HIV-infected men had a greater prevalence and extent of coronary artery plaque than uninfected men.
Caution
· Imaging was not repeated over time.
Implication
· This study supports the hypothesis that HIV infection or its treatment increases the risk for coronary artery disease.
-The Editors
------------------------------
Advances in the treatment of HIV infection have led to dramatic decreases in AIDS-related mortality (1-2). This extension in expected survival has led to the emergence of chronic noninfectious age-related diseases, such as coronary artery disease (CAD) (3). Increased risk for CAD has been associated with HIV infection and antiretroviral therapy (4-6). However, data are not consistent because of differences in populations and study designs (7). For example, the SMART (Strategies for Management of Antiretroviral Therapy) study reported a lower risk for cardiovascular events among persons treated with continuous (vs. intermittent) antiretroviral therapy, possibly mediated by better suppression of HIV RNA levels and systemic inflammation (8).
The incidence of clinical CAD events among HIV-infected persons has been generally low and therefore difficult to study. Subclinical atherosclerosis can be detected noninvasively by using carotid ultrasonography to measure carotid artery intima-media thickness and carotid plaque. Furthermore, noncontrast cardiac computed tomography (CT) can be used to measure coronary artery calcium (CAC) levels. Subclinical atherosclerosis is associated with risk for cardiovascular events in the general population (9). Some studies have found more subclinical atherosclerosis among HIV-infected persons (10-12), but the results are not consistent (13).
Recent advances in technology have allowed for a more comprehensive evaluation of subclinical coronary atherosclerosis by using coronary CT angiography at lower radiation exposure (14). This procedure allows for accurate assessment of the presence and extent of coronary artery plaque, as well as detection of stenosis and characterization of plaque composition. Noncalcified and mixed plaques have been associated with increased risk for plaque rupture and cardiovascular events (15-16). We performed noncontrast cardiac CT scans and CT angiography to determine whether HIV-infected men have more coronary atherosclerosis than uninfected men in the MACS (Multicenter AIDS Cohort Study).
Methods
Population
MACS is an ongoing prospective cohort study of the natural and treated histories of HIV-1 infection in homosexual and bisexual men, conducted in Baltimore, Maryland; Chicago, Illinois; Pittsburgh, Pennsylvania; and Los Angeles, California (17). Initial enrollment in the MACS parent study occurred in 1984 to 1985, with additional enrollment in 1987 to 1991 and 2001 to 2003. The cohort comprises HIV-infected and uninfected men who attend semiannual research visits, which include standardized interviews, physical examinations, and blood and urine collection for laboratory measurements.
Eligibility for this MACS cardiovascular ancillary study included being an active MACS participant (with oversampling of HIV-infected men), being aged 40 to 70 years, weighing less than 136 kg (200 pounds), and having no history of cardiac surgery or percutaneous coronary intervention because these procedures would interfere with the measurement of coronary atherosclerosis. All participants completed noncontrast cardiac CT scanning for CAC scoring between January 2010 and August 2013. Men with atrial fibrillation, chronic kidney disease (estimated glomerular filtration rate <60 mL/min/1.73 m2 during a previous MACS visit), or a history of intravenous contrast allergy were excluded from CT angiography studies. All eligible participants had an estimated glomerular filtration rate greater than 60 mL/min/1.73 m2 within 1 month of CT angiography. The study was approved by the institutional review boards of all participating sites. All participants provided informed consent.
Computed Tomography Scanning and Analysis Procedures
Details of the CT scanning procedures have been described (18). Briefly, in preparation for cardiovascular imaging, men received ß-blocker or calcium-channel blocker medications as needed, and sublingual nitroglycerin was administered before intravenous contrast injection unless contraindicated. Computed tomography scanning equipment included 64-slice multidetector CT at 3 centers and 320-row multidetector CT at 1 center. Prospective electrocardiography-triggering protocols, which minimized radiation exposure, were used unless the heart rate was too fast or irregular. The median dose for the CT angiography procedure was 1.9 mSv (interquartile range, 1.7 to 2.7 mSv).
The CT images were transferred to the core CT reading center (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center) and were analyzed by trained, experienced readers who were blinded to participant characteristics and HIV serostatus. Each segment was analyzed using the modified 15-segment model of the American Heart Association (19). Using axial images, multiplanar reconstructions, and maximum-intensity projections, the reader assessed the presence, size, and composition of coronary plaque and the degree of luminal narrowing stenosis in all assessable coronary segments. Plaque size was graded as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). Segment stenosis was defined as 0 (none), 1 (1% to 29% [minimal]), 2 (30% to 49% [mild]), 3 (50% to 69% [moderate]); or 4 (≥70% stenosis [severe]). The total plaque score was calculated by summing the plaque size score for all assessable coronary segments that showed any plaque (calcified, noncalcified, or mixed), up to a maximum score of 45. This measure has been shown to be highly reproducible (20). The segment involvement score was calculated as the sum of coronary artery segments with plaque, regardless of the degree of stenosis.
Each coronary segment was classified as normal or containing noncalcified, mixed (<50% of plaque area occupied by calcium), or calcified plaque. Calcified atherosclerotic plaque was defined as any structure with attenuation greater than 130 HU visualized separately from the intravascular lumen, identified in at least 2 independent planes. Noncalcified atherosclerotic plaque was defined as any discernible structure that could be clearly assignable to the vessel wall, with a CT density less than the contrast-enhanced coronary lumen but greater than the surrounding connective tissue, and identified in at least 2 independent planes. The noncalcified, mixed, and calcified plaque scores were calculated by summing the plaque scores in each plaque segment separately. Participants and their medical care providers (if permission was obtained) received a clinical CT angiography report. Need for additional testing and therapy was determined by participants' providers.
Clinical Variables
Participants were seen every 6 months as part of routine MACS research visits. Data were collected on CAD risk factors and HIV clinical variables by history, physical examination, and blood tests. For this analysis, we used data that had been collected at the previous MACS visit closest to the CT scan, generally within 6 months. Race or ethnicity was based on self-report. We measured glucose, total and high-density lipoprotein cholesterol, and triglyceride levels from fasting blood samples at the routine MACS visit. Low-density lipoprotein cholesterol level was calculated by using the Friedewald equation or was measured directly in persons with triglyceride levels greater than 4.5 mmol/L (>400 mg/dL) or with nonfasting samples. Serum creatinine level was measured at each MACS visit and within 30 days of CT scanning for persons who had contrast injection. We used the Modification of Diet in Renal Disease equation to estimate glomerular filtration rate. Hypertension was defined as systolic blood pressure greater than 140 mm Hg, diastolic blood pressure greater than 90 mm Hg, or self-reported use of antihypertensive medication. Diabetes mellitus was defined as a fasting serum glucose level 7.0 mmol/L or greater (≥126 mg/dL) or use of medications to treat diabetes. Measures of HIV disease activity in infected men included plasma HIV RNA levels, CD4+ T-cell counts, history of an AIDS-defining cancer or opportunistic infection, and duration of highly active antiretroviral therapy (HAART).
Statistical Analysis
The distributions of demographic and clinical factors in HIV-infected and uninfected men and completion of contrast-enhanced or noncontrast cardiac CT scans were compared by using the Wilcoxon rank-sum test or chi-square test, when appropriate. Because factors associated with the initiation (prevalence) and progression (extent) of plaque may differ, our analytic approach treated each as a separate outcome. Analyses of associations between HIV serostatus and coronary artery plaque prevalence were done with plaque presence defined as a score greater than zero. We used Poisson regression with robust variance (21) to evaluate associations between HIV serostatus and plaque prevalence. Separate analyses were done for each outcome, including CAC; total, calcified, mixed, and noncalcified plaque; and coronary artery stenosis greater than 50%, with minimal adjustment (that is, adjustment for age, race, CT scanning center, and cohort status [enrolled before or after 2001]). Multivariable analyses were then done with additional adjustment for established CAD risk factors (systolic blood pressure, use of hypertension medications, use of diabetes medications, fasting glucose level, total and high-density lipoprotein cholesterol levels, use of lipid-lowering medications, body mass index, and pack-years of tobacco smoking). We used linear regression to assess the association between HIV serostatus and plaque extent (burden) among participants with plaque present (that is, plaque score >0). We used separate models for CAC and total, calcified, mixed, and noncalcified plaque, all of which were adjusted for age, race, CT scanning center, and cohort status. We then adjusted for CAD risk factors as described previously. Because plaque scores were not normally distributed, these values were natural log-transformed. To test for interaction between age and HIV serostatus, interaction terms were added to each model. Significant interactions (P < 0.05) are reported in the Results section. Additional analyses among HIV-infected men were done to assess associations between HIV clinical variables and plaque outcomes (prevalence and extent) by using modified Poisson and linear regression, with adjustment for age, race, CT scanning center, and cohort status (enrolled before or after 2001), followed by adjustment for CAD risk factors. To address possible unmeasured confounding, we performed simple probabilistic sensitivity analyses for selected results (22). For this analysis, we assumed the prevalence of the potential confounder among HIV-infected and uninfected men was sufficiently high, and the association between HIV infection and the unknown confounder was strong. We drew these proportions from a uniform distribution between 0.4 and 0.7 for infected and 0.2 and 0.5 for uninfected men, and we assumed a relative risk between the confounder and disease of 5. To compare the prevalence of noncalcified plaque between HIV-infected and uninfected men in coronary segments with stenosis greater than 50%, we used a log-binomial model with a generalized estimating equation and adjusted for age, race, CT scanning center, and cohort status. Multiple imputation was used for missing CAD risk factor data for multivariable models. For each outcome, the multiple imputation included all predictors and the outcome. Missing values were imputed 5 times on the basis of the distribution of covariates by using a Markov-chain Monte Carlo method (23) assuming multivariable normality. Values were missing and imputed for multiple regression analyses for use of hypertension medications (n = 11), body mass index (n = 27), use of diabetes medications (n = 11), pack-years of smoking (n = 5), use of lipid-lowering medications (n = 20), total and high-density lipoprotein cholesterol levels (n = 28), systolic blood pressure (n = 43), and fasting glucose level (n = 36). All statistical analyses were done using SAS, version 9.2 (SAS Institute, Cary, North Carolina). The SAS procedures used included PROC FREQ, PROC MEANS, PROC NPAR1WAY, PROC MI, PROC MIANALYZE, PROC GENMOD, and PROC REG. The Figure was created by using TIBCO Spotfire S+, version 8.2 (TIBCO Software, Palo Alto, California). Statistical significance was established at a P value less than 0.05.
Results
A total of 1001 men had noncontrast CT scan results available, and 759 of them had coronary CT angiography results; 1 man with a technically limited CAC scan was excluded. The median time between the MACS visit in which covariates were assessed and the CT scan date was 2 months (interquartile range, 1 to 4 months). The study population characteristics are presented in Table 1. The 618 HIV-infected men were slightly younger and more likely to be African American than the 383 uninfected men. More HIV-infected men were current smokers and had greater cumulative pack-years of smoking, lower body mass index, and higher serum creatinine levels. Low- and high-density lipoprotein cholesterol levels were lower in HIV-infected men, and triglyceride levels were higher.
Lipid-lowering therapy was used by approximately one third of all men. Men who had only a noncontrast CT scan had higher serum creatinine levels, were slightly older, had a higher prevalence of diabetes and hypertension, and had lower levels of low-density lipoprotein cholesterol. No discernible differences in the results were seen when the CAC sample was restricted to those who also received contrast scans.
The prevalence of any CAC (Agatston score >0) on noncontrast CT scans was 53.1% among HIV-infected men and 52.0% in uninfected men (Table 2). After adjustment for age, race, CT scanning center, and cohort, there was a greater prevalence of CAC in HIV-infected men (prevalence ratio [PR], 1.21 [95% CI, 1.08 to 1.35]) (Table 3). After additional adjustment for CAD risk factors, this association became borderline significant (PR, 1.12 [CI, 0.99 to 1.26]). Among persons with CAC present on a CT scan, no association existed between HIV serostatus and CAC extent.
Among men who had cardiac CT scans with contrast enhancement, the prevalence of plaque in any coronary segment was 77.6% for HIV-infected men and 74.4% for uninfected men. After adjustment for age, race, CT scanning center, and cohort, there was a greater prevalence of plaque in HIV-infected men than in uninfected men (PR, 1.14 [CI, 1.05 to 1.24]), which persisted after adjustment for CAD risk factors (PR, 1.13 [CI, 1.04 to 1.23]). Plaque extent was measured as total plaque and segment involvement scores. Both of these scores were greater among HIV-infected men (P = 0.009 and 0.023, respectively), but differences were attenuated and only borderline significant after adjustment for CAD risk factors (P = 0.062 and 0.075, respectively). The HIV association with the aforementioned scores differed slightly by race. The scores were higher among white HIV-infected men but not among African Americans (data not shown).
Additional analyses were done to determine the association between HIV serostatus and plaque composition on CT angiography (noncalcified, mixed, or calcified plaque). More HIV-infected than uninfected men (63.3% vs. 53.1%) had noncalcified plaque. This higher prevalence persisted after adjustment for age, race, CT scanning center, and cohort (PR, 1.28 [CI, 1.13 to 1.45]) and remained significant after adjustment for additional CAD risk factors (PR, 1.25 [CI, 1.10 to 1.43]). To address possible unmeasured confounding, we performed simple probabilistic sensitivity analyses (22). Even in the presence of strong confounding, the significant association between noncalcified plaque and HIV serostatus remained. Among men with noncalcified plaque, there was also a significant association between the extent of noncalcified plaque and HIV infection (mean difference in log plaque score, 0.15 [CI, 0.02 to 0.29] for HIV-infected compared with uninfected men after full adjustment).
In both HIV-infected and uninfected men, mixed and calcified plaque were less prevalent than noncalcified plaque (Table 2). Mixed plaque was more prevalent in HIV-infected than uninfected men (PR, 1.35 [CI, 1.10 to 1.65]). This association was attenuated and borderline significant after we adjusted for CAD risk factors (PR, 1.22 [CI, 0.98 to 1.52]). Among men with mixed plaque, there was no association between HIV serostatus and the extent of mixed plaque. We also saw no association between HIV serostatus and the presence or extent of calcified plaque on CT angiography (Table 3).
The prevalence of coronary artery stenosis greater than 50% in any coronary segment was 16.9% among HIV-infected men and 14.6% among uninfected men (PR, 1.48 [CI, 1.06 to 2.07]) after adjustment for age, race, CT scanning center, and cohort. This association was no longer statistically significant after adjustment for CAD risk factors (PR, 1.23 [CI, 0.86 to 1.75]). Among 121 men, 192 coronary segments showed 50% or greater stenosis on CT angiography. Of these segments, the proportion of noncalcified plaque in HIV-infected men was 45% compared with 35% in uninfected men (P = 0.23 [adjusted for age, race, CT scanning center, and cohort and controlled for within-person correlation]). We repeated the analyses in Table 3 and included only men with suppressed HIV RNA, and the results were nearly identical to those from the entire cohort.
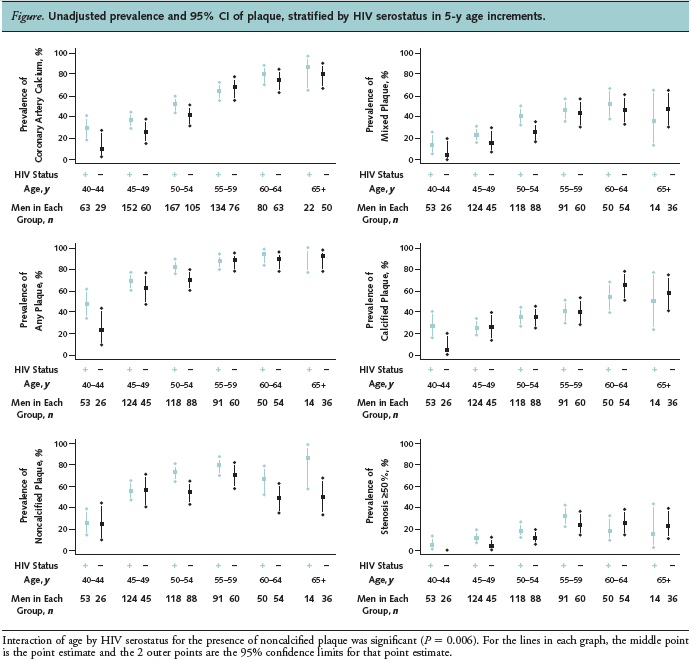
The unadjusted prevalence and 95% CI of each plaque type stratified by HIV serostatus and age are shown in the Figure. Advancing age was associated with an increase in prevalence of coronary artery plaque and stenosis; however, we noted a significant interaction between age and HIV serostatus for the presence of noncalcified plaque. Advancing age was associated with noncalcified plaque in HIV-infected men (PR per 5-year increase in age, 1.17 [CI, 1.11 to 1.23]) but not in uninfected men (PR per 5-year increase in age, 1.03 [CI, 0.96 to 1.11] P for interaction = 0.006).
We examined HIV-related clinical stage-of-disease variables to evaluate associations with subclinical atherosclerosis that might contribute to a greater prevalence and extent of some types of coronary artery plaque among HIV-infected men. The presence of CAC or any plaque on CT angiography was not significantly associated with detectable plasma HIV RNA levels, duration of HAART, history of AIDS, or current or nadir CD4+ T-cell count (P > 0.10 for each) in minimally or fully adjusted models. Higher nadir CD4+ cell count was associated with lower prevalence of noncalcified plaque (PR, 0.95 [CI, 0.91 to 1.00]) in the fully adjusted model. Significant associations were seen with the extent of plaque and HIV clinical variables. The extent of CAC was positively associated with the presence of a detectable HIV RNA level (mean difference in log plaque score, 0.56 [CI, 0.05 to 1.06]), and mixed plaque extent was positively associated with history of AIDS (mean difference in log plaque score, 0.38 [CI, 0.03 to 0.73]) in fully adjusted models. No other significant associations were seen between HIV clinical variables and the extent of plaque.
In contrast, several HIV clinical variables were associated with the presence of coronary artery stenosis greater than 50% (Table 4), which was not attenuated with the inclusion of traditional CAD risk factors. Longer duration of HAART and lower nadir CD4+ T-cell count were associated with the presence of coronary artery stenosis greater than 50% in separate regression models. When both of these factors were placed in the same model, the associations all remained significantly associated with stenosis greater than 50% (PR per year of HAART, 1.08 [CI, 1.01 to 1.16]; PR per 100-cell increase in nadir CD4+ T-cell count, 0.82 [CI, 0.70 to 0.96]). We saw similar results when the analyses were limited to men with suppressed HIV RNA. Although the prevalence of 50% or greater stenosis was higher (PR, 1.4) among those with detectable viral load or a history of AIDS, these associations were not statistically significant. No association was seen with current CD4+ T-cell count.
Discussion
In MACS, we found a greater prevalence and extent of coronary atherosclerosis on coronary CT angiography among HIV-infected men than among uninfected men. Coronary CT angiography allows for identification of specific plaque composition. Noncalcified plaque prevalence and extent were each independently and positively associated with HIV-positive serostatus even after adjustment for CAD risk factors. Noncalcified plaques may be more prone to rupture, leading to acute coronary syndromes (15-16). However, in this cohort, relatively few coronary clinical events have been identified to date. Among HIV-infected men, coronary artery stenosis greater than 50% was associated with lower nadir CD4+ T-cell count and longer treatment with HAART.
Of note, MACS includes a control group of uninfected men drawn from the same reference population with a similar lifestyle-men who have sex with men-and this helps to minimize unmeasured potential confounding and selection bias. A smaller study of 102 HIV-infected men and women and 41 uninfected control participants also found more coronary segments containing noncalcified plaque among HIV-infected patients (24). The results of our larger study confirm a greater prevalence and extent of noncalcified plaque among HIV-infected men. The larger sample size allowed for sufficient power to also identify a significant interaction by age for noncalcified plaque. The prevalence of noncalcified plaque increased with advancing age in HIV-infected men but not in uninfected men, which is also consistent with the higher prevalence in infected men that is seen as early as age 50 years (Figure). Additional studies that include repeated longitudinal assessments of plaque in this cohort are needed to determine whether noncalcified plaques calcify at a slower rate in HIV-infected men or whether the incidence of new noncalcified plaques is increased.
We found positive associations between the presence of coronary artery stenosis greater than 50% and both a lower nadir CD4+ T-cell count and greater years of HAART. Low CD4+ T-cell count has been associated with carotid intima-media thickness (10) and carotid artery plaque (25), although these associations were not seen in some other studies (26). The SMART study showed that continuous use of antiretroviral therapy reduced CAD events compared with interrupted antiretroviral therapy (8). Coronary artery stenosis greater than 50% is an indication of advanced atherosclerosis. Lower nadir CD4+ T-cell count is probably a marker of longer duration of uncontrolled viremia in years before the initiation of effective antiretroviral therapy. In addition, lower nadir CD4+ T-cell count and longer duration of HAART are markers of longer duration of HIV infection and exposure to older formulations of antiretroviral therapy, with greater potential adverse metabolic effects, although controlling for traditional CAD risk factors accounts for some of these effects. Our future studies will investigate associations between coronary atherosclerosis with specific antiretroviral therapy regimens and measures of inflammation and immune activation.
Our study has important strengths, including the use of coronary CT angiography, which allows for detailed assessment of subclinical coronary atherosclerosis. We also had a large, ethnically diverse participant population from a well-characterized cohort that includes an appropriate HIV-uninfected control group. Limitations include the cross-sectional study design, inclusion of men only, and limitations inherent to observational studies. We saw differences in some cardiovascular disease risk factors between HIV-infected and uninfected men (for example, more HIV-infected men reported smoking). We performed detailed assessment of potential confounders and analyses with and without adjustment for CAD risk factors. Therefore, the differences in plaque by HIV infection status that persisted are not attributable to these population confounders. Some risk factor data were missing for a few men and were therefore imputed. We also performed risk factor-adjusted analyses that excluded these persons, and the results were essentially unchanged. Finally, for the outcome for which we found pronounced differences (noncalcified plaque), we performed sensitivity analyses and determined that unmeasured confounding would have to be extremely strong to attenuate the observed association.
The assessment of an association with noncalcified plaque was possible because of the use of contrast-enhanced CT scans. Noncalcified plaque cannot be identified on noncontrast cardiac CT scans, which are used to measure CAC levels. Coronary artery calcium is known to be a potent predictor of CAD events in the general population, and a CAC score of zero is generally associated with very low risk for a CAD event (9). Future studies are needed to assess this risk in patients with HIV who may have more noncalcified plaque. Computed tomography angiography can also differentiate mixed plaque (plaque that has <50% calcification) from calcified plaque. The latter may reflect more advanced stable atherosclerosis, whereas the former, like noncalcified plaque, may be more prone to rupture. Because this study is cross-sectional, future studies are needed to determine whether there are differences in the progression of the atherosclerotic process in patients with HIV, which might lead to a greater prevalence of noncalcified plaque.
In conclusion, noncalcified coronary artery plaque was more prevalent and extensive in HIV-infected men, suggesting increased risk for cardiovascular events. Men with more advanced HIV infection, which is shown by low nadir CD4+ T-cell count and a greater number of years receiving HAART, have a higher prevalence of clinically significant coronary stenosis greater than 50%. Additional studies are needed to identify how best to prevent progression of atherosclerosis in this unique population and the correlation of atherosclerosis with future events. Although coronary CT angiography is not indicated as a screening test in asymptomatic persons, these results emphasize the importance of assessing and modifying traditional cardiovascular risk factors in this population, especially in men with a history of low nadir CD4+ T-cell count.
|
|
| |
| |
|
|
|