| |
Is bone loss linked to chronic inflammation in antiretroviral-naive HIV-infected adults? A 48-week matched cohort study.
|
| |
| |
Download the PDF here
AIDS Ahead of print post acceptance May 26 2014
Hileman, Corrilynn O.; Labbato, Danielle E.; Storer, Norma J.; Tangpricha, Vin; McComsey, Grace A.
"In conclusion, HIV-infected, ART-naive adults, but not controls, had BMD loss at the total hip and trochanter sites over this 48-week, matched, prospective cohort study, although changes in BMD did not reach statistical significance between the groups. The HIV-infected individuals were more likely to have bone loss at the trochanter site and this may be related to heightened inflammation. Further, higher baseline IL-6 was associated with progression to osteopenia or osteoporosis in the HIV-infected group. 25(OH)D concentration at baseline was not associated with baseline or change in BMD in this study. Therapeutics targeting inflammation may benefit bone health in HIV-infected adults naive to ART and further study in this area is needed."
Abstract
Objective: Antiretroviral therapy (ART) has been implicated in bone loss in HIV. The role of inflammation and vitamin D is unclear and better investigated in ART-naive individuals.
Design and methods: This is a 48-week, prospective cohort study to compare baseline and change in hip and spine bone mineral density (BMD) measured by dual-energy X-ray absorptiometry in HIV-infected, ART-naive adults and healthy controls matched by age, sex, and race. We also studied associations between bone loss and inflammation markers and plasma 25-hydroxyvitamin D [25(OH)D] using logistic regression.
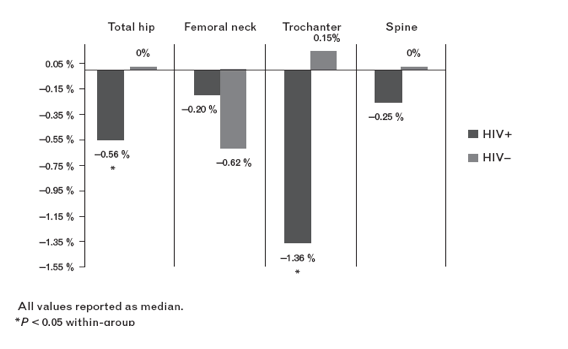
Results: Forty-seven HIV-infected adults and 41 controls were included. Baseline 25(OH)D, BMD at total hip, trochanter, and spine, and prevalence of osteopenia and osteoporosis were similar between groups. In the HIV-infected group, total hip and trochanter, but not spine, BMD decreased over 48 weeks [hip -0.005 (-0.026-0.008) g/cm2, P = 0.02 within group; trochanter -0.013 (-0.03-0.003), P < 0.01]. BMD did not change at any site within controls. The HIV-infected group was more likely to have bone loss at the trochanter (P = 0.03). This risk persisted after adjustment for age, sex, race, BMI, smoking, and hepatitis C (odds ratio 4, 95% confidence interval 1.2-15.8). In the HIV-infected group, higher interleukin-6 concentrations (P = 0.04) and Caucasian race (P < 0.01) were independently associated with progression to osteopenia or osteoporosis, but not 25(OH)D levels.
Conclusion: BMD at the total hip and trochanter sites decreased in the HIV-infected, ART-naive adults, but not controls, over this 48-week study. Higher serum interleukin-6 concentrations were associated with progression to osteopenia or osteoporosis status in the HIV-infected group.
In comparing the proportion of participants with bone loss at each site, the HIV-infected group was 2.8 times more likely than controls to have bone loss at the trochanter site [73 vs. 49% for HIV-infected vs. controls; OR 2.8, 95% confidence interval (CI) 1.1-7.2, P1/40.034]. This risk persisted after adjustment for age, sex, race, BMI, smoking, and hepatitis C (OR 4.3, 95% CI 1.2-15.8, P=0.026). In an exploratory analysis to assess if heightened inflammation or vitamin D status was in the causal pathway between HIV and bone loss at the trochanter site, adding IL-6, sTNFR-II, sVCAM-1, or sICAM-1 attenuated the OR for HIV status by greater than 10%. Further, with each of these inflammatory markers in the model, HIV status was no longer independently predictive of bone loss at the trochanter site (Table 5). Taken together, this suggests that heightened inflammation is in the causal pathway and is a possible explanation for why HIV status was associated with this outcome.
In case of HIV-infected participants, 20.5% progressed from normal bone to osteopenia or from osteopenia to osteoporosis vs. 5.6% of controls (P1/40.089). In the HIV-Association infected group, higher baseline IL-6 (OR 1.1, 95% CI 1-1.2, P=0.036) and Caucasian race (OR 17.4, 95% CI 2.1-142, P=0.008) were independently associated with this outcome in multivariable modeling. Other inflammatory markers, 25(OH)D level and HIV-related factors including baseline CD4? T-cell count were not independently associated with this outcome.
.......although vitamin D deficiency was common in both groups in this study, vitamin D status was not associated with change in BMD.......Potential explanations include the fact that African American race, which has been shown to effect the relationship between 25(OH)D and BMD [39,40], was the most predominant race in this study. Also, it was recently recognized that in the general population, measurements of free 25(OH)D, and not just total 25(OH)D, are better correlated with BMD [41]. Lastly, most of the participants in this study - HIV infected and controls - were vitamin D-deficient, so we were unable to compare change in BMD with a vitamin D-sufficient group. Ongoing vitamin D supplementation trials will be able to establish a causal link in the relationship between vitamin D status and BMD in HIV.
DISCUSSION excerpts
In this study, we have comprehensively assessed change in BMD and the association with inflammation and vitamin D status in HIV-infected adults naive to ARTwith an age, sex, and race-matched healthy control group for comparison. Similar to previous studies [8,11,15], the prevalence of low BMD in the ART-naive HIV-infected group at baseline was high (36%); however, it was similar to what was seen in the matched controls (32%). Low baseline BMD in this study was associated with traditional osteoporosis risk factors including older age, low BMI, and female sex. Over 48 weeks, BMD decreased significantly at both the total hip and trochanter sites in the HIV-infected group, but again the changes in BMD were not statistically different than matched healthy controls. However, after adjustment for traditional
osteoporosis risk factors, the ART-naive HIV-infected adults were more likely to have bone loss at the trochanter site than controls, and this risk appeared to be associated with heightened inflammation. Also, progression from normal bone to osteopenia or from osteopenia to osteoporosis was independently associated with higher baseline IL-6 levels in the HIV-infected group.
It has been suggested that the process of bone resorption and bone formation (or bone remodeling), which is normally a tightly regulated balance, is uncoupled in HIV [26]. HIV viral proteins have been shown to directly stimulate osteoclast activity [27] and suppress osteoblast activity [28]. Further, TNF-a and IL-6, inflammatory cytokines known to be elevated in both ART-treated and untreated-HIV-infected individuals compared to uninfected controls [29,30], both promote osteoclast formation [31-33]. In this study, given the suggestion that systemic inflammation contributes to the risk of bone loss at the trochanter site, perhaps with longer follow-up, greater differences would be seen with regard to change in BMD over time. A major strength of this study was the large number of ART-naive HIV-infected individuals who remained ART-naive through 48 weeks of followup, allowing the opportunity to study the effect of HIV on bone without the confounding effect of ART. However, with current ART treatment guidelines recommending ART initiation at any CD4 T-cell count, longer duration of follow-up was not feasible. Indeed, over half (24/40) of the study participants had initiated ART within 1 year after completion of this 48-week study.
Fig. 1. Percentage change in bone mineral density at each site by group. All values reported as median.P<0.05 within group.
|
|
| |
| |
|
|
|