| |
Adherence/how many Tenofovir PrEP pills worked in study - Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study.....iPrEx open-label extension
|
| |
| |
Download the PDF here
The Lancet Infectious Diseases, September 2014
Robert M Grant, Peter L Anderson, Vanessa McMahan, Albert Liu, K Rivet Amico, Megha Mehrotra, Sybil Hosek, Carlos Mosquera, Martin Casapia,
Orlando Montoya, Susan Buchbinder, Valdilea G Veloso, Kenneth Mayer, Suwat Chariyalertsak, Linda-Gail Bekker, Esper G Kallas, Mauro Schechter,
Juan Guanira, Lane Bushman, David N Burns, James F Rooney, David V Glidden, for the iPrEx study team
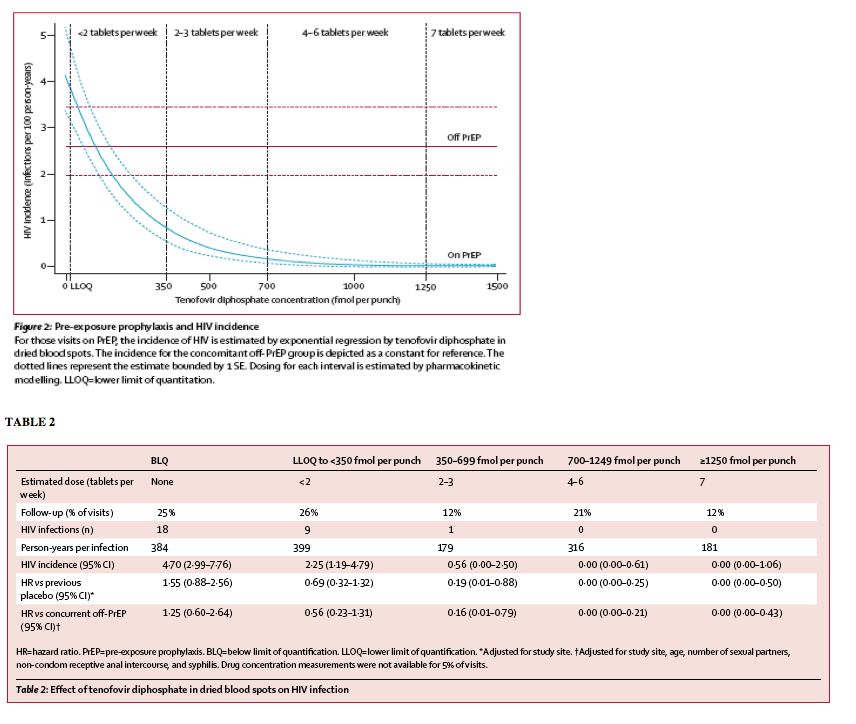
"Drug concentrations in dried blood spots were strongly associated with HIV incidence among those receiving PrEP (figure 2). There were no infections at visits where tenofovir diphosphate concentration was 700 fmol per punch or greater, suggesting the use of four to seven tablets per week (table 2). Such protective drug concentrations were evident during 33% of visits among those receiving PrEP. The hazard ratio for infection was 0·00 (95% CI 0·00-0·17) compared with the previous placebo group and 0·00 (0·00-0·14; adjusted for baseline differences in HIV risk factors) compared with concurrent off-PrEP group. The dried blood spot concentration associated with 90% reduced risk of HIV acquisition relative to the off-PrEP group was 611 fmol per punch (95% CI 216-1006), consistent with use of two or three tablets per week."
"Uptake of PrEP was high across a range of demographic subgroups of men and transgender women who have sex with men who were previously enrolled in masked placebo-controlled trials, and had access to PrEP at no charge from experienced health-care providers. Such high uptake has also been reported among heterosexual couples finishing the placebo-controlled phase of the Partner's PrEP trial.21 These findings contrast with population surveys of men who have sex with men suggesting that use of PrEP is still low overall,22, 23 with barriers including low awareness of PrEP,23 lack of knowledge and experience among health-care providers,24 and ambiguity about whether PrEP should be provided by HIV specialists or general practitioners.25 Removing these barriers to access, as occurred in this study and other settings,26showed substantial demand for PrEP. Concerns about the safety of antiretroviral drugs was the most common reason for declining PrEP among those with ready access. Although most public discussion of PrEP has focused on efficacy and adherence, information about safety that was confirmed in trials is important for all considering PrEP.
People who more often engaged in risky sexual practices and who had sexually transmitted infections were more likely to join the study, more likely to choose PrEP, and more likely to have sustained protective levels of PrEP use. Such preferential use of PrEP during times of greater risk is expected to increase the effect and cost-effectiveness of PrEP services, and shows people's capacity to recognise and respond appropriately to risks when given attractive options. Access to PrEP was associated with a roughly 50% reduction in HIV incidence compared with concurrent and historical controls. The lower concentrations of tenofovir disphophate among transgender women might be a result of lower adherence or different pharmacokinetics; more information is needed (panel)."
"The overall protection conferred by PrEP was strongly associated with a long-term measure of cumulative PrEP dosing, tenofovir diphosphate in red blood cells measured with dried blood spots. The concentration of drug in dried blood spots associated with a 90% reduction in HIV incidence corresponded to use of two or three tablets per week; this estimate is consistent with dose-effect relationships reported during the randomised phase of iPrEx.4 Although oral PrEP with tenofovir disoproxil fumarate and emtricitabine is recommended for daily use, which helps foster dosing habits, the drug concentrations achieved with daily dosing (tenofovir diphosphate ≥1250 fmol per punch) were substantially higher than the protective threshold for men and transgender women who have sex with men, providing protection against HIV innfection even if a few doses are missed. This relation between drug concentrations in blood and protection from HIV apply to this cohort for whom anal intercourse was the primary risk factor; the minimum required adherence to PrEP and the relation between blood drug concentrations and protection from vaginal or other viral exposure might be different."
Summary
Background
The effect of HIV pre-exposure prophylaxis (PrEP) depends on uptake, adherence, and sexual practices. We aimed to assess these factors in a cohort of HIV-negative people at risk of infection.
Methods
In our cohort study, men and transgender women who have sex with men previously enrolled in PrEP trials (ATN 082, iPrEx, and US Safety Study) were enrolled in a 72 week open-label extension. We measured drug concentrations in plasma and dried blood spots in seroconverters and a random sample of seronegative participants. We assessed PrEP uptake, adherence, sexual practices, and HIV incidence. Statistical methods included Poisson models, comparison of proportions, and generalised estimating equations.
Findings
We enrolled 1603 HIV-negative people, of whom 1225 (76%) received PrEP.
Uptake was higher among those reporting condomless receptive anal intercourse (416/519 [81%] vs 809/1084 [75%], p=0·003) and having serological evidence of herpes (612/791 [77%] vs 613/812 [75%] p=0·03).
Of those receiving PrEP, HIV incidence was 1·8 infections per 100 person-years, compared with 2·6 infections per 100 person-years in those who concurrently did not choose PrEP (HR 0·51, 95% CI 0·26-1·01, adjusted for sexual behaviours), and 3·9 infections per 100 person-years in the placebo group of the previous randomised phase (HR 0·49, 95% CI 0·31-0·77). Among those receiving PrEP, HIV incidence was 4·7 infections per 100 person-years if drug was not detected in dried blood spots, 2·3 infections per 100 person-years if drug concentrations suggested use of fewer than two tablets per week, 0·6 per 100 person-years for use of two to three tablets per week, and 0·0 per 100 person-years for use of four or more tablets per week (p<0·0001).
PrEP drug concentrations were higher among people of older age, with more schooling, who reported non-condom receptive anal intercourse, who had more sexual partners, and who had a history of syphilis or herpes.
Interpretation
PrEP uptake was high when made available free of charge by experienced providers. The effect of PrEP is increased by greater uptake and adherence during periods of higher risk. Drug concentrations in dried blood spots are strongly correlated with protective benefit.
Funding
US National Institutes of Health.
Introduction
Pre-exposure prophylaxis (PrEP) with oral emtricitabine and tenofovir disoproxil fumarate prevents the acquisition of HIV among men and transgender women who have sex with men,1 heterosexual couples,2 and heterosexual men and women.3 The effectiveness of PrEP depends greatly on both the efficacy of the drugs4, 5 and multiple social interactions and behaviours related to uptake and adherence.
In randomised placebo-controlled trials,1, 2 adherence to PrEP (assessed by detection of drugs in blood) was a strong correlate of efficacy. HIV risk was reduced by 90% or more among people using PrEP who had detectable drug in two trials,4, 5 whereas two trials of African women showed no evidence of efficacy on an intention-to-treat basis; despite high reported adherence, less than a third of participants receiving active drug had detectable concentrations in their blood.6, 7
The theory of risk compensation predicts that people are more likely to participate in risky sexual practices with the advent of biomedical disease-prevention strategies, including medical circumcision, antiretroviral treatment for HIV infection, and PrEP.8 By contrast, self-reported sexual practices became safer in trials of PrEP,2, 9, 10 including among people who thought that they were receiving the active treatment and that it would be effective.9 Self-reported increases in safe behaviour were corroborated by decreases in the incidence of syphilis and prevalence of acute HIV infection.9
Patterns in PrEP use11 and sexual practices12 could differ in clinical practice from those reported in clinical trials. Participants in masked and placebo-controlled efficacy trials are informed that they might be receiving a placebo or a drug with no benefit and that product safety requires further confirmation. Such messages could undermine adherence and limit risk compensation. As information from trials about PrEP safety and efficacy becomes available, adherence could increase and condom use could decrease. Open-label treatment could also alter uptake of PrEP; as people focus more on their personal goals rather than research goals, intentions to use PrEP might be greater when HIV exposure is greatest or PrEP might be taken up in clinical practice primarily by the so-called worried well, who are already protecting themselves in other ways. The overall effect of PrEP in practice depends on these behaviors.
Our aim was to investigate PrEP uptake, adherence, and sexual practices in a way that more closely resembles clinical practice. Because social desirability can bias self-reported adherence, we use tenofovir diphosphate measured in dried blood spots as a novel biomarker of long-term PrEP use.
Discussion
Uptake of PrEP was high across a range of demographic subgroups of men and transgender women who have sex with men who were previously enrolled in masked placebo-controlled trials, and had access to PrEP at no charge from experienced health-care providers. Such high uptake has also been reported among heterosexual couples finishing the placebo-controlled phase of the Partner's PrEP trial.21 These findings contrast with population surveys of men who have sex with men suggesting that use of PrEP is still low overall,22, 23 with barriers including low awareness of PrEP,23 lack of knowledge and experience among health-care providers,24 and ambiguity about whether PrEP should be provided by HIV specialists or general practitioners.25 Removing these barriers to access, as occurred in this study and other settings,26showed substantial demand for PrEP. Concerns about the safety of antiretroviral drugs was the most common reason for declining PrEP among those with ready access. Although most public discussion of PrEP has focused on efficacy and adherence, information about safety that was confirmed in trials is important for all considering PrEP.
People who more often engaged in risky sexual practices and who had sexually transmitted infections were more likely to join the study, more likely to choose PrEP, and more likely to have sustained protective levels of PrEP use. Such preferential use of PrEP during times of greater risk is expected to increase the effect and cost-effectiveness of PrEP services, and shows people's capacity to recognise and respond appropriately to risks when given attractive options. Access to PrEP was associated with a roughly 50% reduction in HIV incidence compared with concurrent and historical controls. The lower concentrations of tenofovir disphophate among transgender women might be a result of lower adherence or different pharmacokinetics; more information is needed (panel).
----------------------
Panel
Research in context
Systematic review
We searched PubMed on June 30, 2014, with the terms "preexposure prophylaxis and HIV" or "tenofovir, HIV, and prevention". This search yielded 630 publications. There were primary reports of randomised clinical trials of pre-exposure HIV prophylaxis (nine publications and one conference abstract), surveys of people's interest in using pre-exposure prophylaxis if it were available, and behavioural surveys of self-reported pre-exposure prophylaxis use. We did not identify any longitudinal studies of uptake and adherence to open-label pre-exposure prophylaxis. US Centers for Disease Control and Prevention guidelines recommend daily pre-exposure prophylaxis with oral emtricitabine and tenofovir disoproxil fumarate for HIV-uninfected adults at substantial risk of HIV, including men who have sex with men who, in the past 6 months, have had condomless receptive anal intercourse with more than one partner or a sexually transmitted infection, or heterosexual men and women who have had condomless intercourse with partners known to be at substantial risk for HIV, or any adult with a sexual partner who is HIV negative.27 Transgender women are not mentioned in these guidelines. Despite the US Food and Drug Administration's approval of pre-exposure prophylaxis and these broad recommendations by the Centers for Disease Control and Prevention, overall self-reported use of pre-exposure prophylaxis has been low in surveys (less than 5%). Barriers to uptake include low levels of awareness among people at risk for HIV, lack of information about pre-exposure prophylaxis among potential providers, ambiguities as to whether pre-exposure prophylaxis should be provided by HIV specialists or general practitioners, concerns about insurance coverage and co-payments, and provider concerns about risk compensation.
Interpretation
Our study shows that uptake is high when barriers to PrEP supply are eliminated. Uptake and adherence were higher among people reporting higher sexual risk of acquiring HIV infection rather than among the so-called worried well. The pre-exposure prophylaxis cascade shows substantial discontinuation of prophylaxis after initiation, despite the paucity of side-effects, especially among younger people. A substantial portion of people with no detectable drug in blood plasma had previously experimented with pre-exposure prophylaxis as suggested by analysis of dried blood spots. Drug concentrations in dried blood spots strongly correlated with pre-exposure prophylaxis protection, with no HIV infections occurring if drug concentrations suggested use of four or more tablets per week over long periods. More information is needed about PrEP adherence and pharmacokinetics in transgender women. Self-reported sexual practices became safer in the cohort, irrespective of whether pre-exposure prophylaxis was used; the lack of risk compensation is corroborated by similar syphilis incidence among users and non-users of pre-exposure prophylaxis. Making pre-exposure prophylaxis available had a substantial effect on HIV transmission in populations with a disproportionate burden of the epidemic.
------------------------
The percentage of PrEP users with detectable drug concentrations increased substantially among Peruvian men after the release of information about efficacy and safety from randomised trials. The Peruvian men in the study were young and racially diverse, and comprised the majority of participants in this study.
Sustained engagement is a significant challenge for PrEP services. In this open-label extension, by contrast with the randomised phase of the study, we inquired about desire to start or stop PrEP at every visit and explored perceptions of risks and preferences. Disengagement from PrEP services was substantial and HIV infection rates during gaps in PrEP use were high. Among those who stop PrEP, disengagement typically occurred early after a brief period of experimentation with PrEP. The causes for disengagement were not identified for most participants: side-effects and toxic effects were rare. Substance and alcohol use were not associated with disengagement. Although some young people sustained effective PrEP use, drug concentrations and overall retention were lower with younger age. Although low adherence among young people is affected by developing neurocognitive capacities28 and social development in emerging adulthood,29 age-related social and structural characteristics probably contributed to this finding, possibly involving daily concomitant use of other drugs, age parity with clinic staff, income, employment, housing, and stigma. New ways to attract and engage younger men and transgender women who have sex with men are needed, especially in view of the high incidence of HIV in this group.
The overall protection conferred by PrEP was strongly associated with a long-term measure of cumulative PrEP dosing, tenofovir diphosphate in red blood cells measured with dried blood spots. The concentration of drug in dried blood spots associated with a 90% reduction in HIV incidence corresponded to use of two or three tablets per week; this estimate is consistent with dose-effect relationships reported during the randomised phase of iPrEx.4 Although oral PrEP with tenofovir disoproxil fumarate and emtricitabine is recommended for daily use, which helps foster dosing habits, the drug concentrations achieved with daily dosing (tenofovir diphosphate ≥1250 fmol per punch) were substantially higher than the protective threshold for men and transgender women who have sex with men, providing protection against HIV innfection even if a few doses are missed. This relation between drug concentrations in blood and protection from HIV apply to this cohort for whom anal intercourse was the primary risk factor; the minimum required adherence to PrEP and the relation between blood drug concentrations and protection from vaginal or other viral exposure might be different.
Reporting results of plasma drug testing to PrEP users was well accepted: those informed of positive results appreciated the validation of their adherence efforts, and those informed of negative results were not surprised.30 Participants often asked for quantitative measurements of drug concentrations, so sharing information from dried blood spot analysis might be an attractive way to reinforce and troubleshoot adherence, especially now that levels of protection associated with different drug concentrations have been established for men and transgender women who have sex with men. Analysis of drug concentrations in hair could have the same advantages.31
We recorded no evidence of risk compensation during open-label access to PrEP. Sexual practices became safer by self-report and syphilis incidence was not greater among PrEP users. PrEP use among heterosexual couples in Africa also showed no change in sexual practices with HIV-infected partners.32 Although PrEP might serve as a daily reminder of imminent risk, we noted similar trends toward safer reported behaviour among PrEP users and non-users, suggesting that cohort participation and access to comprehensive prevention services were stronger drivers of these behavioural trends. Making PrEP available provided several indirect benefits, including engagement of people at risk, HIV testing, identification of HIV infection including some acute infections, diagnosis and treatment of sexually transmitted infections, and longer-term counselling. The direct benefits of providing PrEP included a substantial reduction in HIV transmission among men and transgender women who have sex with men, including high-level protection among active users.
Results
We enrolled participants between June 13, 2011, and June 26, 2012. Most patients came from the randomised phase of the iPrEx study, which had the last treatment visits in November, 2010 (figure 1). Former participants of ATN 082 completed treatment visits in November, 2010, and were enrolled in Chicago and were all non-white men aged 18-25 years. Former participants of US Safety Study ended treatment visits in 2009 and enrolled in San Francisco and Boston. 265 (17%) of 1603 who enrolled and were eligible for PrEP were white, 125 (8%) of 1603 were black, 73 (5%) of 1603 were Asian, 1063 (70%) of 1603 were mixed or other ethnic origin, and 1094 (72%) of 1603 were Latino. Of those who were eligible for PrEP, people who enrolled in the open-label extension compared with those who did not tended to be older (mean age 28 years vs 26 years, p<0·0001), more likely to report non-condom receptive anal intercourse at their original screening visit (911/1526 [60%] vs 447/814 [55%], p=0·03), more likely to have a history of syphilis (222/1526 [15%] vs 80/814 [10%], p=0·001) or herpes (578/1526 [38%] vs 246/814 [30%] p<0·0001), and were similar in schooling, ethnic origin, transactional sex, and identification as transgender. Participation in the open-label extension was much the same among those formerly assigned to placebo and active treatment groups in the previous randomised studies (data not shown).
Of the 446 people who had been in the active treatment group of iPrEx, and for whom drug testing had been done at week 8, those with detectable drug were more likely to enrol in the open-label extension than were those without detectable drug (191/277 [69%] vs 93/169 [57%] after weighting for sample probability, p=0·02). Of 814 eligible iPrEx participants who did not enrol in the open-label extension, 344 (42%) were last seen before the end of the randomised treatment phase of iPrEx, 192 (24%) were last seen during the post-treatment phase of the randomised study, and 278 (34%) were last seen at the unmasking visit that happened shortly before the open-label extension began enrolment at each site.
1230 (77%) of 1603 people wanted to receive PrEP according to the computer-based self-interview. Reasons for not requesting PrEP were concern about side-effects (185/373, 50%), not wanting to take a pill every day (59/373, 16%), not liking taking pills (47/373, 13%), preference for other prevention methods (52/373, 14%), fear that people will think they have HIV (26/373, 7%), and fear that people will know they have sex with men or transgender people (11/373, 3%). The reasons for declining PrEP did not differ significantly by previous randomisation group (data not shown).
Acute HIV infection was clinically suspected in 30 participants, of whom two (7%) were subsequently noted to have detectable HIV RNA. HIV RNA testing was negative for the other 28, of whom 25 started PrEP after an average delay of 44 days (range 9-136). PrEP was dispensed to 1225 (76%) of 1603 participants, including 1128 (72%) of 1573 asymptomatic seronegative participants who received PrEP at the enrolment visit, and 97 (6%) of all 1603 seronegative patients who received PrEP at a later visit. Receipt of PrEP was significantly higher among those who reported non-condom receptive anal intercourse and had serological evidence of herpes simplex virus 2 infection at the open-label extension enrolment (table 1). PrEP uptake was not associated with previous treatment-group randomisation, age, education, alcohol or substance use, or country.
Blood plasma was tested for tenofovir at week 4 (n=305), week 8 (n=851), or week 12 (n=33); 36 were not tested. Of all people who received PrEP and who had blood plasma tested, drug was detected in 847 (71%), and varied by study region: 185 (83%) of 222 in the USA, 142 of 185 (77%) in Brazil, 357 (63%) of 538 in Peru, 93 (62%) of 150 in Ecuador, 27 (68%) of 40 in South Africa, and 43 (80%) of 54 in Thailand. The proportion of patients with tenofovir in blood plasma was similar in the open-label extension and in the first 8 weeks of the randomised phase of the iPrEx trial (149/213 [70%, or 60% after weighting for sampling fraction], p=0·09). In 63 participants who were tested during both phases of the iPrEx study, drug detection increased in Peru (the country with the most PrEP recipients) from 44% (28/63) in the randomised phase to 63% (40/63) in the open-label extension (p=0·02), and was much the same at other sites (data not shown).
Two participants were RNA positive at the time of enrolment; both were clinically suspected to have HIV and PrEP was not started. Furthermore, 41 people were infected with HIV during the study; 13 of those not receiving PrEP (2·6 infections per 100 person-years, 95% CI 1·5-4·5) and 28 of those receiving PrEP (1·8 infections per 100 person-years, 95% CI 1·3-2·6). The clinic had stopped dispensing PrEP to seven people more than 2 months before seroconversion because of side-effects noted by the provider (hypersensitivity in one patient, gastritis in another), side-effects noted by the user (dizziness, nausea, and flatulence in one patient, weight gain in another), loss to follow-up (n=1), and participant preference (n=2). Of those receiving PrEP, HIV incidence was 49% (95% CI -1 to 74) lower than among those who did not choose PrEP after adjusting for the higher risk sexual practices at baseline among PrEP users, and 36% lower before adjustment (95% CI -24 to 67%). Considering only participants from iPrEx, the HIV incidence on PrEP was 53% (95% CI 26 to 70) lower than in the placebo group of the randomised phase (3·93 infections per 100 patient-years) and 51% (95% CI 23 to 69) lower than during the gap between the randomised phase and the open-label extension (3·81 infections per 100 person-years).
Because adherence to PrEP is an important determinant of efficacy, sensitive indicators of long-term use of PrEP are needed. Tenofovir diphosphate concentrations were detected in dried blood spots from 70 of 92 participants (77% weighted for sampling) who had no detectable drug in blood plasma at week 8, showing the higher sensitivity of dried blood spot analysis. Drug was detected in dried blood spots in one (2%) of 60 people who never received PrEP in the open-label extension; the participant had previously been randomly assigned to the active group of iPrEx and had not returned all pill bottles.
Drug concentrations in dried blood spots were strongly associated with HIV incidence among those receiving PrEP (figure 2). There were no infections at visits where tenofovir diphosphate concentration was 700 fmol per punch or greater, suggesting the use of four to seven tablets per week (table 2). Such protective drug concentrations were evident during 33% of visits among those receiving PrEP. The hazard ratio for infection was 0·00 (95% CI 0·00-0·17) compared with the previous placebo group and 0·00 (0·00-0·14; adjusted for baseline differences in HIV risk factors) compared with concurrent off-PrEP group. The dried blood spot concentration associated with 90% reduced risk of HIV acquisition relative to the off-PrEP group was 611 fmol per punch (95% CI 216-1006), consistent with use of two or three tablets per week.
Drug concentrations were higher among participants of older age, with more years of schooling, non-condom receptive anal intercourse, more sexual partners, a history of syphilis or herpes, any HIV-positive sexual partner, or lower estimated creatinine clearance (table 3). The effect of age was not explained by differences in estimated creatinine clearance and was distributed across the range of ages (table 3). Drug concentration in dried blood spots was not associated with alcohol use, methamphetamine use, or cocaine use (table 3). Concentration of drug in dried blood spots at week 4 was associated with sustained use of PrEP over time (figure 3). The most common pattern of PrEP use was clinically significant use (≥350 fmol per punch) with subsequent discontinuation; intermittent use (with periods of starting and stopping) was not common (figure 3). HIV infection occurred during gaps in PrEP use: at the time of the first laboratory evidence of HIV infection, drug concentrations were 350 fmol per punch or greater for one of 28 (4%) of seroconverters and 442 of 1338 (33%) controls (figure 3, p<0·0001). The proportion having such drug concentrations was much the same among HIV seroconverters and seronegative controls early during PrEP use, decreased over time in both groups (p<0·0001 for decreasing proportions in each group over time), and decreased more rapidly among seroconverters (p=0·02 for the proportion decreasing more rapidly among seroconverters than among seronegative participants; figure 3).
For PrEP to be effective, it needs to be taken during periods of risk for HIV acquisition and adhered to. At enrolment, 1054 (67%) of 1573 eligible HIV-negative participants without a viral syndrome had indications for PrEP as defined as non-condom receptive anal intercourse, more than one anal intercourse partner, or a recent sexually transmitted infection (syphilis, gonorrhoea, or chlamydia diagnosed at the visit). Of that group, 793 (75%) chose to use PrEP. Of those at-risk who started PrEP, 736 (93%) returned at week 12, of whom 688 (93%) were still being dispensed PrEP. Of those still being dispensed PrEP, 583 (85%) reported taking PrEP within the past 3 days, of whom 111 (70%) of the 158 who were tested had clinically significant drug concentrations in dried blood spots. Extrapolating from the sample with drug test results, 409 (39%) of 1054 who had HIV risk at baseline had clinically significant PrEP use through to week 12. Retention in the study was not associated with receipt of PrEP (945/1128 [84%] vs 312/378 [83%], p=0·42). Older people were less likely to miss a visit (defined as more than 4 months between visits irrespective of drug detection; p=0·006). Retention was not related to non-condom receptive anal intercourse (p=0·95), having five or more sexual partners in the past 6 months (p=0·92), or being transgender (p=0·82).
PrEP treatment was interrupted 380 times among 365 participants for reasons other than loss to follow-up, end of the study, or HIV infection. These interruptions represented 15·6% of the time after starting PrEP. The reasons for interruptions were participant preference (151/380 times, 6·6% of follow-up), side-effects (93/380 times, 3·7% of follow-up), effects of a significant but unrelated comorbidity (38/380 times, 1·1% of follow-up), relocation or travel (52/380 times, 2·4% of follow-up), and other (53/380 times, 1·8% of follow-up). Other causes included suspected acute HIV infection (8/380 times, 0·2% of follow-up) and recent sexual exposure deemed to warrant a three-drug regimen for postexposure prophylaxis (15/380 times, 0·1% of follow-up). Gastrointestinal symptoms, such as nausea or abdominal pain, were the most common symptoms leading to interruption of PrEP. There were three confirmed increases in serum creatinine concentration, all grade 1, all returned to baseline after stopping PrEP, and none recurred after restarting PrEP. One seroconverter on PrEP had the reverse transcriptase Met184Val mutation associated with resistance to emtricitabine.
Self-reported total number of sexual partners, non-condom receptive anal intercourse, and non-condom insertive anal intercourse, all decreased during follow-up in the group receiving PrEP and in the group not receiving PrEP. The proportion reporting non-condom receptive anal intercourse decreased from 34% (377/1115) to 25% (232/926) among PrEP recipients (p=0·006), and from 27% (101/369) to 20% (61/304) among non-recipients (p=0·03). The decrease in non-condom receptive anal intercourse, non-condom insertive anal intercourse, and numbers of sexual partners were much the same in the each groups (p=0·95, p=0·56, p=0·64, respectively). Syphilis incidence was similar among PrEP recipients and non-recipients (7·2 infections per 100 patient-years vs 5·4 infections per 100 patient-years, HR 1·35, 95 CI 0·83-2·19).
Methods
Participants
In this cohort study we sought to identify demographic and behavioural characteristics associated with PrEP uptake and adherence and the effect of PrEP uptake and adherence on HIV incidence and sexual practices. We enrolled participants from three previous randomised controlled trials: ATN 082,13 iPrEx,1 and US Safety Study.14All participants in the iPrEx open-label extension were designated male at birth, reported having had anal intercourse with men, were older than age 18 years, and had previously participated in a randomised masked placebo-controlled trial of once daily oral PrEP with emtricitabine and tenofovir disoproxil fumarate (iPrEx or ATN 082) or tenofovir disoproxil fumarate only (US Safety Study). Participants who were identified as infected with HIV during the randomised phases of previous trials were followed up, although they were not eligible for PrEP; they are not included in this report.
All participants provided written informed consent. The open-label extension protocol was approved by ethical committees governing each study site and by national regulatory authorities in each country, including registration with the US Food and Drug Administration.
Procedures
Participants were told their randomised assignment before enrolment in the open-label extension. After providing informed consent and before HIV testing, patients answered a computer questionnaire to assess desire to use PrEP, reasons for declining PrEP (selected from a list as all that apply), self-identification as trans (selected a list of identities as all that apply, translated according to local custom), education, alcohol use (in the past 30 days), and controlled substance use (in the past 30 days). At the enrolment visit, all participants were offered daily oral PrEP with emtricitabine and tenofovir disoproxil fumarate if they were HIV-antibody negative and they had no symptoms of acute HIV infection. For those with an acute viral syndrome, PrEP was deferred until HIV RNA testing was negative or HIV antibody testing continued to be negative after resolution of symptoms. All benefits of study participation were provided irrespective of whether participants chose to take PrEP; such benefits varied by study site in accordance with local standards and ethical committee requirements. Visits were done at enrolment and at weeks 4, 8, 12, 24, 36, 48, 60, and 72. Participants could start PrEP on any visit during the first 48 weeks of follow-up, and were followed up at weeks 4, 8, and 12 after starting PrEP then every 12 weeks until completing a total of 72 weeks on study (off or on PrEP). Counselling support included integrated next-step counselling,15, 16 which involved counselling for sexual health for all participants and PrEP adherence assessment and counselling for those receiving PrEP. All participants were informed that the results of PrEP drug testing would be shared with them; results were provided by a medical officer. Results from drug testing done during previous randomised trials were not provided to the study sites or to the study participants.
We assessed drug concentrations in blood plasma for all participants at one of their study visits during the first 12 weeks after receiving PrEP. Drug concentrations in dried blood spots were measured for participants who opted to receive PrEP using a case-cohort design.17 This design tested all timepoints after PrEP dispensation among those with confirmed HIV infection and a site-stratified sample of seronegative participants. Roughly 27% of seronegative participants were selected with a pseudorandom number list, overseen by the study statistician (DVG). Analyses of drug concentrations were weighted inversely to the probability of selection for testing. Only results from dried blood spots were used in the analysis of correlates of drug detection.
Patients were tested for HIV antibodies at all visits and tested for syphilis, herpes, and urethritis every 24 weeks or if they had symptoms. Two rapid tests were used for HIV testing, with western blot testing to confirm any reactive test result.1 PrEP was discontinued at the time of any reactive test, and resumed if confirmatory tests were negative. Blood plasma (with EDTA) was drawn and dried blood spots were prepared at enrolment and all 12-week follow-up visits irrespective of receipt of PrEP. Plasma and dried blood spots were also collected 4 weeks and 8 weeks after starting PrEP. Dried blood spots were stored at -20°C within 24 h of collection and shipped on dry ice to the laboratory where 3 mm punches were taken and analysed for tenofovir diphosphate by liquid chromatography and tandem mass-spectroscopy, as previously described.18, 19 We estimated creatinine clearance with the Cockcroft-Gault equation.
We estimated dosing from the tenofovir diphosphate concentration using pharmacokinetic modelling from observations of drug accumulation and decay after 1 month of daily dosing.18 Tenofovir diphosphate in dried blood spots has a half-life of 17 days, corresponding with a 25-fold accumulation with daily dosing. The lower limit of quantitation of the assay was 2·5 fmol per punch.19 Dosing categories were below lower limit of quantitation, lower limit of quantitation to 349 fmol per punch (fewer than two tablets per week), 350-699 fmol per punch (two or three tablets per week), 700-1249 fmol per punch (four to six tablets per week), and 1250 fmol per punch or more (daily dosing).
Statistical analysis
We compared HIV incidence on and off PrEP with a Poisson model (with a robust SE), enabling us to compare the randomised and open-label periods. We measured HIV incidence by tenofovir diphosphate concentration in dried blood spots with profile likelihood CIs because of the small number of infections in each dosing category. We estimated the concentration of drug associated with 90% protection as a relative risk of 0·10 compared with the concurrent off-PrEP group, after adjustment for non-condom receptive anal intercourse, age, number of partners, history of syphilis, and enrolment site.4 We assessed predictors of drug concentrations, by dosing category, with an ordinal logistic regression model20 with a robust SE, adjusted for study site and time on study. We compared sexual practices on PrEP with generalised estimating equations.
Role of the funding source
The study was sponsored by the US National Institutes of Health, which had input into the study design and the analysis of the data, but no role in data collection, data interpretation, or writing of the report. Study drug was donated by Gilead Sciences, which did not have input into any part of the study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
|
|
| |
| |
|
|
|