| |
PrEP & PEP Update - SF recent news, NYS Plan announced during summer 2014, rectal/vaginal gels & future long-acting PrEP (injectables, vaginal rings) update from HIVR4P Conference
|
| |
| |
S.F. men shed condoms in favor of Gilead's HIV prevention pill (Dec 8, 2014).....http://www.bizjournals.com/sanfrancisco/blog/biotech/2014/12/hiv-aids-prep-truvada-condom-gilead-gild.html?page=all.......Among those taking PrEP, he said, there has been no increase in the number of their sexual partners and no new HIV infections........disappearing stigma around PrEP use......one-third of those referred to Kaiser's program choose not to start PrEP after speaking with a counselor
Early Experiences Implementing Pre-exposure Prophylaxis (PrEP) for HIV Prevention in San Francisco (PLOS Medicine, March 2014)......"As interest in PrEP among high seroincidence populations continues to rise in San Francisco, additional PrEP delivery sites in the community are needed".......http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.1001613......PrEP in an STD clinic- From September 2012-2013, 571 individuals have been evaluated at SFCC (San Francisco City Clinic) for PrEP, and 261 participants have enrolled in The Demo Project, for an overall uptake of 49% among potentially eligible clients.......Since the launch of the Kaiser's PrEP program in San Francisco in April 2012, more than half of the 123 men and women referred by both HIV-specialty providers (65%) and non-HIV-specialty providers (35%) have initiated PrEP (table 2).......PrEP in an HIV-specific reproductive health program (While only a few women have initiated PrEP at BAPAC thus far, data suggest HIV-serodifferent couples in the US want to incorporate PrEP as a safer conception option)......Bay Area Perinatal AIDS Center (BAPAC) is a program of the University of California San Francisco, providing comprehensive preconception management and prenatal care to HIV-positive women and HIV-negative women with HIV-positive male partners......In 2010, BAPAC began offering PrEP to HIV-negative pregnant women who were having sex without a condom with HIV-positive male partners. In 2012, BAPAC expanded the PrEP program to include HIV-uninfected women seeking safer conception options with their HIV-infected male partners. BAPAC has also recently launched the PRO-Men initiative to educate HIV-positive men who have sex with women about their reproductive options and provide preventive services to their HIV-negative female partners, including PrEP. BAPAC provides care for women through six weeks post partum, when women transition to a primary care provider who can continue to prescribe PrEP as needed.
NYS PrEP Announcement from AIDS Institute (click here for details)
Coming Out of the PrEP
Closet.....http://www.huffingtonpost.com/scott-wiener/coming-out-of-the-prep-closet_b_5832370.html
PrEP Article in New York Magazine published July 13 2014/ new WHO Recommendation/ new CDC Recommendation - (07/16/14)
Rectal/Vaginal PrEP at HIVR4P Conference/South Africa Oct 28-31 2014 - (11/03/14) .....Preclinical Evaluation of TMC-278 LA.....Cabotegravir (GSK1265744) Long-acting Injectable Nanosuspension........Tenofovir Intravaginal Rings.......Rectal Gels Containing Maraviroc and/or Tenofovir
2 PrEP Studies IPERGAY & PROUD Announce Efficacy - (11/03/14)
Sex on PrEP (Intl AIDS Conference-Melbourne/July/2014).......To better understand sexual behavior in the context of PrEP, we undertook a qualitative study of sexual experiences among iPrEx OLE participants in the US......http://www.natap.org/2014/IAC/IAC_91.htm
PrEP at CROI, Uptake - MSM, IDUs, Meth Use, Long-Acting
PrEP......http://www.natap.org/2014/CROI/croi_177.htm
Next/2nd Generation PrEP for Men & Women: intravaginal rings, injectables, long-acting, rectal gel, intermittent PrEP (CROI/2012).......http://www.natap.org/2012/CROI/croi_159.htm
Longitudinal Trends in HIV Non-Occupational Post-Exposure Prophylaxis (NPEP) at a Boston Community Health Center Between 1997 and 2013 -(IDSA) (10/23/14)........Many patients in this study demonstrated ongoing high-risk behavior, as manifested by recurrent NPEP and a high HIV incidence. Simpler and better-tolerated regimens are needed to optimize regimen completion. NPEP recipients may benefit from early HIV risk-reduction counseling and pre-exposure prophylaxis.......
Three Quarters of NPEP Users Show Interest in Trying PrEP
High Rate of NPEP Completion, Good Safety, With Raltegravir Plus TDF/FTC
Raltegravir Containing regimen, for Post Exposure Prophylaxis (PEP) well tolerated in Health Care Workers (HCW)
Tolerability, adherence and completion of new occupational post-exposure prophylaxis regimen (tenofovir + emtricitabine and raltegravir)
NYS - UPDATE: HIV Prophylaxis Following Non-Occupational Exposure PEP
Updated: October 2014
NYS Guidelines Post-Exposure Prophylaxis
http://www.hivguidelines.org/clinical-guidelines/post-exposure-prophylaxis/
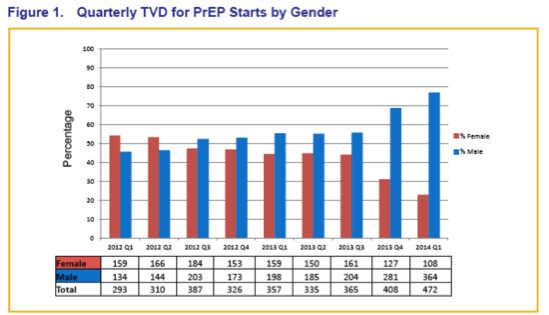
Two Years of Truvada for Pre-Exposure Prophylaxis Utilization in the United States (Glasgow/2014).......http://www.natap.org/2014/GLASGOW/GLASGOW_10.htm
|
|
| |
| |
|
|
|