 |
 |
 |
| |
GSK Long-Acting ART Integrase inhibitor -
Cabotegravir Injected Every 1, 2, or 3
Months Yields Adequate Troughs in Simulation
|
| |
| |
ICAAC 2014. September 5-9, 2014. Washington, DC
Mark Mascolini
Monthly, bimonthly, or quarterly injections of the long-acting integrase inhibitor cabotegravir yielded trough concentrations above the proposed targets for HIV treatment or prevention in simulation studies based on phase 1 and 2 clinical trial data [1]. Gender and body mass index affected cabotegravir absorption rate constant.
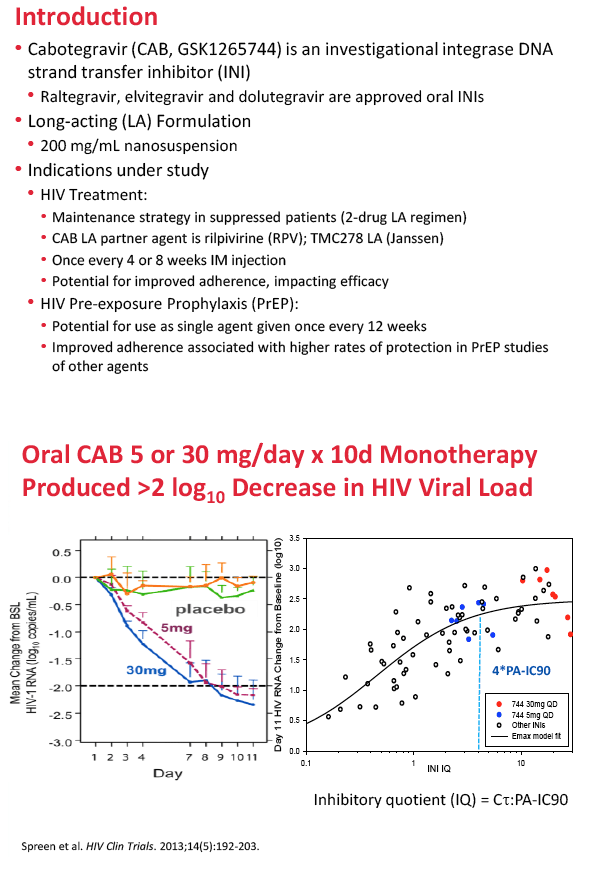
Cabotegravir (GSK1265744) is an integrase inhibitor being developed in oral and long-acting injectable formulations for treatment and prevention of HIV infection [2-5]. In 56 healthy volunteers, a single injection at various test doses yielded half-lives ranging from 21 to 50 days [3]. Two doses of the long-acting injectable agent protected eight macaques from repeated rectal challenge with simian HIV [4].
Cabotegravir is not encased in a nanoparticle. Cabotegravir itself, the actual drug crystal, is milled in order to reduce the drug crystal size to an average of 200 nanometer. Thereafter, it is formulated into an aqueous suspension for injection, but never encased in some other nanoparticle. They use the inherent low aqueous solubility of the drug crystal to control its release (i.e., absorption) from injection site into systemic circulation. It is important to make this distinction as some nanoparticle-based medicines do require other approaches that introduce added compounds/excipients to create the final formulation (with additional toxicology study requirements, etc.).
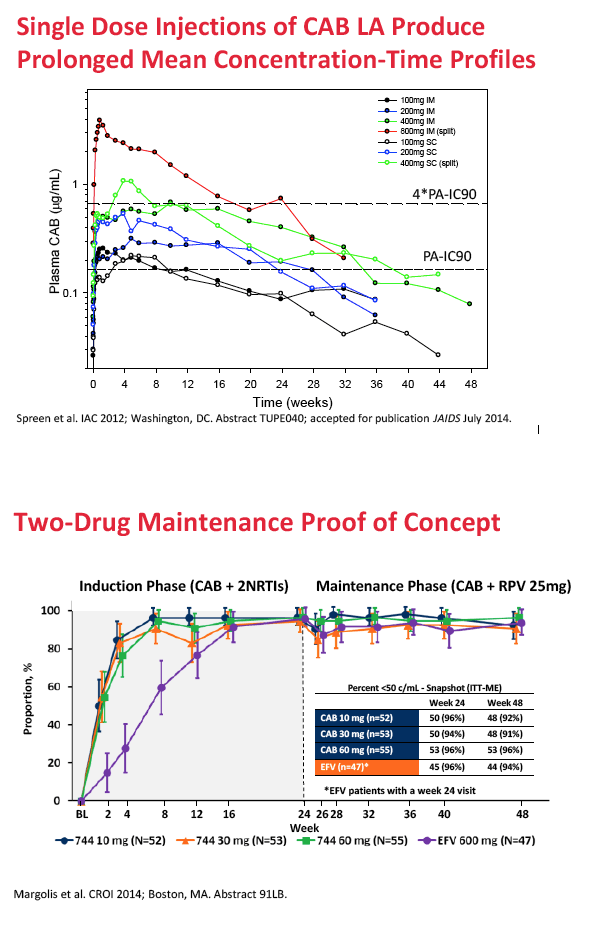
For HIV treatment, ViiV Healthcare is studying cabotegravir as part of a two-drug maintenance regimen administered every 4 to 8 weeks by intramuscular injection with Janssen's long-acting nonnucleoside, rilpivirine. For preexposure prophylaxis (PrEP), ViiV is developing cabotegravir as a single agent given once every 12 weeks.
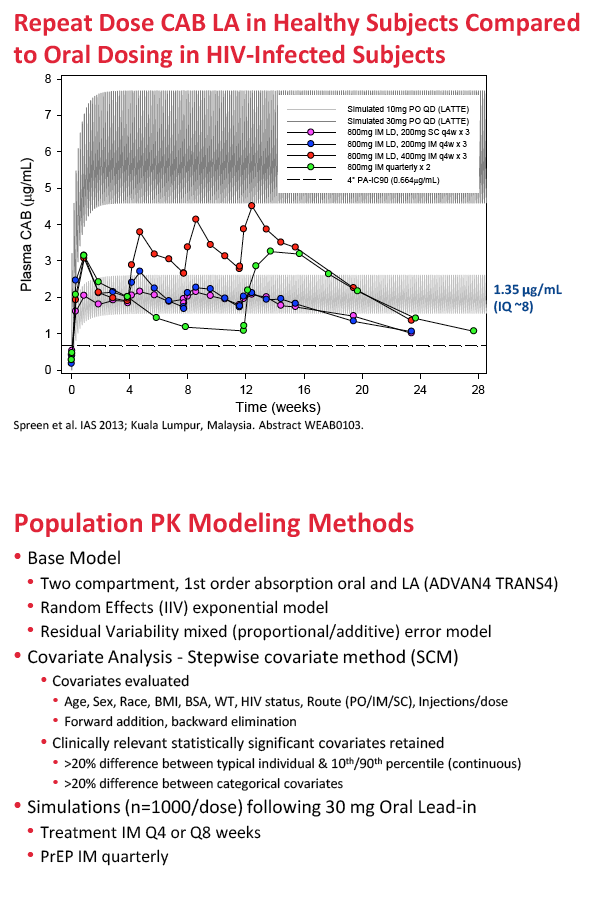
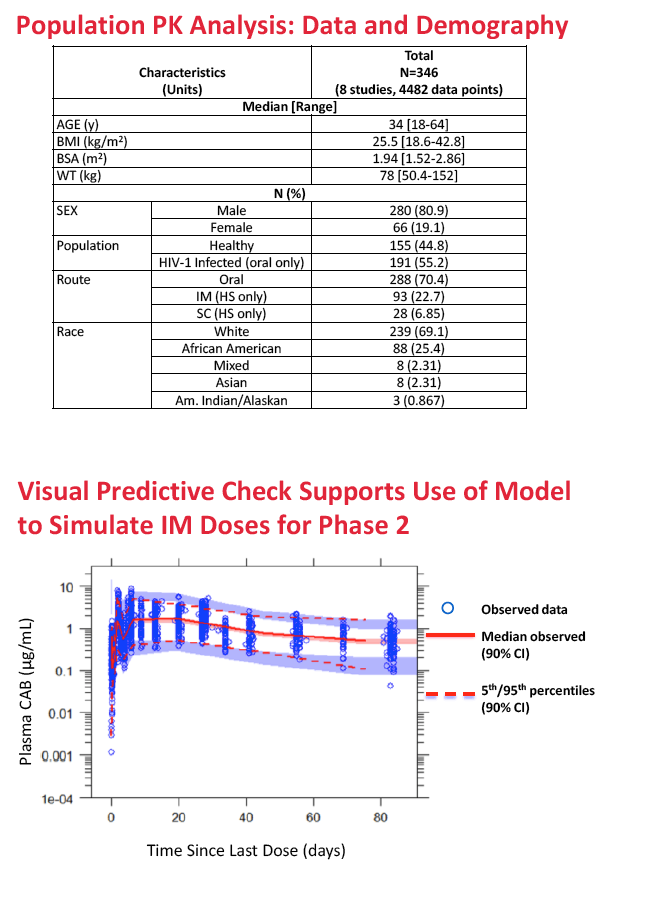
For the new analysis, ViiV Healthcare investigators collected data from eight phase 1 and 2 trials of oral or injectable cabotegravir. They developed a series of models to describe population pharmacokinetics of cabotegravir, aiming toward a final model to simulate long-acting dosing regimens for phase 2b trials. Covariates evaluated included age, sex, race, body mass index, body surface area, weight, HIV status, route of administration, and injections per dose. Simulations assessed intramuscular dosing after a 30-mg oral lead-in dose for (1) treatment every 4 or 8 weeks, and (2) PrEP quarterly.
The population pharmacokinetic analysis involved 4482 observations of 346 trial participants with a median age of 34, a median body mass index of 25.5 kg/m2, and a median weight of 78 kg. Participants included 280 men and 66 women; there were 191 HIV-positive participants and 155 healthy controls. Two thirds were white, and one quarter was African American.
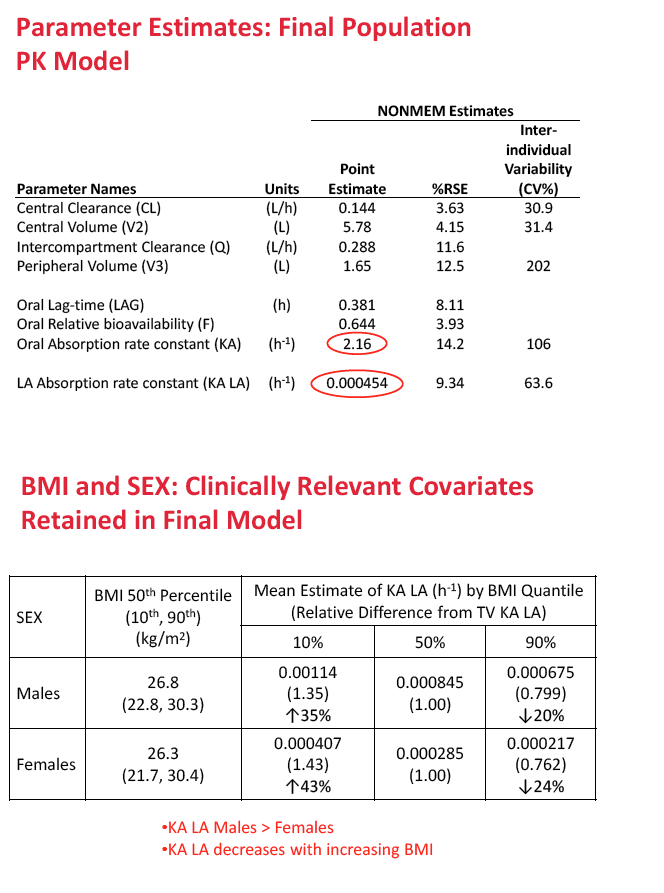
A two-compartment first-order absorption model described both oral and injectable formulations of the integrase inhibitor. In the final population PK model, oral absorption rate constant (KA) was 2.16 h(-1) and long-acting absorption rate constant 0.000454 h(-1). The researchers retained two statistically significant and clinically relevant variables in the final model: (1) sex and (2) body mass index (BMI) effects on the absorption rate constant of injectable cabotegravir.
Compared with median BMI, a BMI above the 90th percentile reduced cabotegravir absorption rate constant 20% in men and 24% in women, while a BMI below the 10th percentile increased cabotegravir absorption rate constant 35% in men and 43% in women. Absorption differences were not seen after oral administration of cabotegravir. The researchers concluded that absorption rate constant is greater in men than women and that absorption rate constant decreases with increasing BMI.
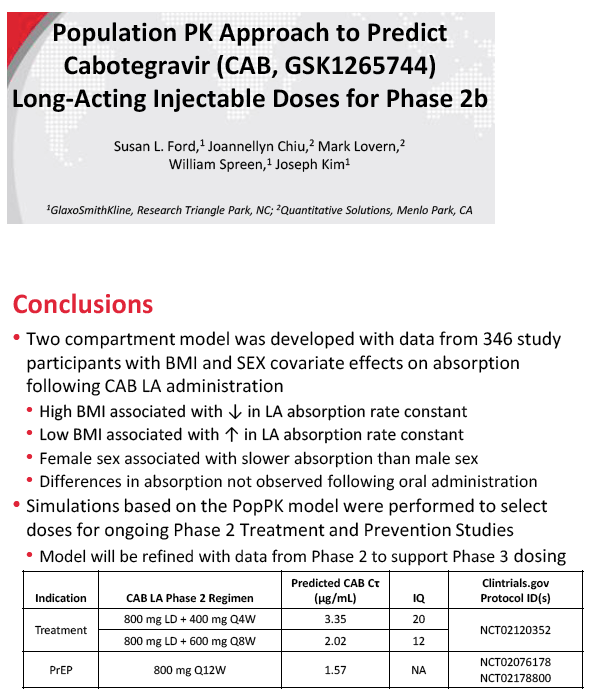
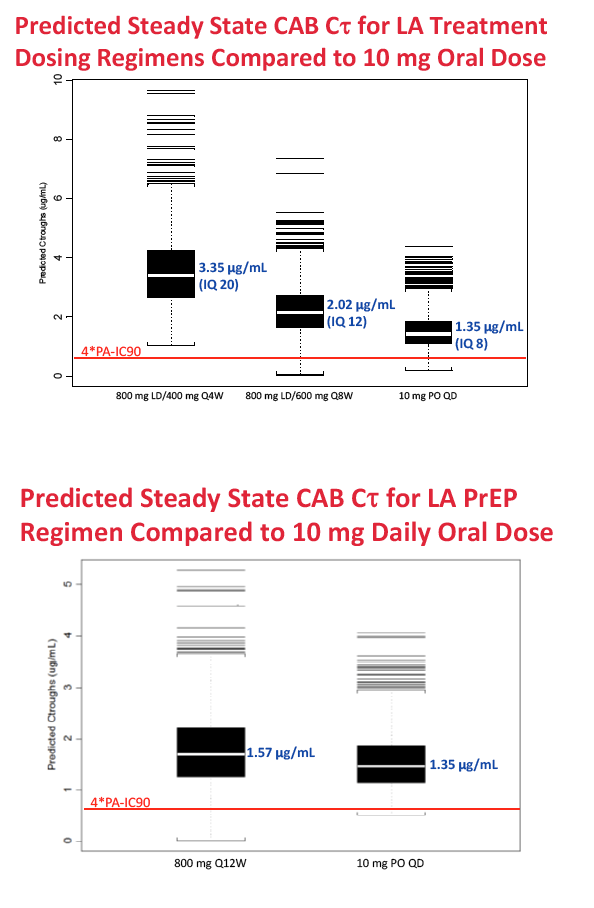
For long-acting treatment regimens, simulation of an 800-mg intramuscular cabotegravir loading dose, followed by 400 mg monthly, predicted an average steady-state cabotegravir trough of 3.35 ug/mL. An 800-mg intramuscular loading dose followed by 600 mg every 2 months predicted an average steady-state trough of 2.02 ug/mL. In comparison, steady-state cabotegravir trough with a 10-mg oral dose averaged 1.35 ug/mL.
For long-acting PrEP, an 800-mg quarterly intramuscular dose yielded an average steady-state cabotegravir trough of 1.57 ug/mL, compared with 1.35 ug/mL with the 10-mg oral dose. Steady-state concentrations with the four cabotegravir doses for treatment or PrEP are all above the proposed target concentrations for HIV treatment and prevention with cabotegravir.
Clinical trials are planned to assess long-acting cabotegravir for treatment (ClinicalTrials.gov identifier NCT02120352) and PrEP (ClinicalTrials.gov identifiers NCT02076178 and NCT02178800). The ViiV team will refine the PK model with phase 2 data to support phase 3 dosing.
References
1. Ford S, Chiu J, Lovern M, et al. Population PK approach to predict cabotegravir (CAB, GSK1265744) long-acting injectable doses for phase 2b (Ph2b). ICAAC 2014. September 5-9, 2014. Washington, DC. Abstract H-645.
2. AIDSinfo. Cabotegravir. AIDSinfo Drug Database. http://aidsinfo.nih.gov/drugs/513/s-gsk1265744/0/patient
2. Ford SL, Margolis D, Chen S, Gould E, Spreen W. Plasma and tissue GSK1265744 pharmacokinetics following long-acting parenteral administration in healthy male and female subjects. 14th International Workshop on Clinical Pharmacology of HIV Therapy, April 22-24, 2013, Amsterdam. Abstract O_02. http://www.natap.org/2013/Pharm/Pharm_06.htm
3. Spreen W, Ford SL, Chen S, et al. Pharmacokinetics, safety and tolerability of the HIV integrase inhibitor S/GSK1265744 long acting parenteral nanosuspension following single dose administration to healthy adults. XIX International AIDS Conference. July 22-27, 2012. Washington, DC. Poster TUPE040.
4. Andrews C, Gettie A, Russell ¬Lodrigue K, et al. Long-acting parenteral formulation of GSK1265744 protects macaques against repeated intrarectal challenges with SHIV. 20th Conference on Retroviruses and Opportunistic Infections. March 3-6, 2013. Atlanta. Abstract 24LB. http://www.natap.org/2013/CROI/croi_10.htm
5. Spreen W, Min S, Ford SL, et al. Pharmacokinetics, safety, and monotherapy antiviral activity of GSK1265744, an HIV integrase strand transfer inhibitor. HIV Clin Trials. 2013;14:192-203. http://www.ncbi.nlm.nih.gov/pubmed/24144896
Program Abstract:
Background: CAB is an integrase inhibitor in development as a long-acting (LA) injectable formulation for treatment and prevention of HIV. Oral CAB demonstrated antiviral efficacy following 10-day monotherapy and long term administration in combination with background ART at daily doses of 5 to 60mg. In healthy subjects, single LA injections of 100 to 800mg maintained plasma CAB concentrations (CONC) up to 52 weeks, and repeat monthly and quarterly injections achieved plasma CONC associated with antiviral activity following oral administration. Population PK (PPK) analysis was used to characterize CAB PK and support LA dose selection for Ph2b trials.
Methods: Data from eight Phase 1 and 2a/b studies of CAB administered orally or by LA injection were included. PPK analysis was conducted using NONMEM (v.7.2) to determine the base structural model with fixed and random effects. Stepwise covariate model building was performed to identify and incorporate significant covariates. Bootstrapping and predictive checks were conducted using PsN (v.3.7.6). The final model was used to simulate LA dosing regimens for Ph2b studies. Results: A total of 346 subjects and 4482 observations were included in the PPK analysis. Both oral and LA formulations were well described by a two-compartment 1st order absorption model, with a lag-time for the oral formulation. An exponential model for random effects and a mixed error model for residual variability were included in the base model. Three statistically significant, clinically relevant covariates were retained in the final model: SEX and BMI effects on LA absorption rate constant (KA LA) and route effect (oral, LA) on central volume. KA LA was reduced 20% and 24% with BMI >30, and increased 35% and 43% with BMI <22-23, in males and females respectively, relative to median BMI. Simulations of CAB LA 800mg IM loading dose followed by 400mg monthly, 600mg bimonthly or 800mg quarterly achieved mean steady state trough CONC of 3.48ug/mL, 2.26ug/mL, or 1.76ug/mL, respectively, all above proposed targets for HIV treatment and prevention.
Conclusions: BMI and SEX were predictors of KA LA. Simulated CAB LA regimens achieved mean trough CONC above therapeutic targets, supporting their administration in Ph2b trials of HIV suppression in infected subjects and prevention in uninfected subjects.
|
| |
|
 |
 |
|
|