 |
 |
 |
| |
Resistance Analysis in Patients Receiving Daclatasvir in Combination With Asunaprevir and BMS-791325: Hepatitis C Virus Genotype 1 Infection
|
| |
| |
Reported by Jules Levin
15th International Workshop on Clinical Pharmacology of HIV and Hepatitis
Therapy. Washington DC, USA, May 19-21, 2014
Hernandez D1, Yu F1, Ueland J1, Vellucci V1, Zhou N1, Yang X1, Hartman-Neumann S1, Han Z1, Falk P1, Chen C1, Sims K2, McPhee F1
1Bristol-Myers Squibb, Wallingford, CT; 2Bristol-Myers Squibb, Hopewell, NJ 08534
Efficacy of an Interferon- and Ribavirin-Free Regimen of Daclatasvir, Asunaprevir, and BMS-791325 in Treatment-Naive Patients With HCV Genotype 1 Infection - (01/31/14) publication
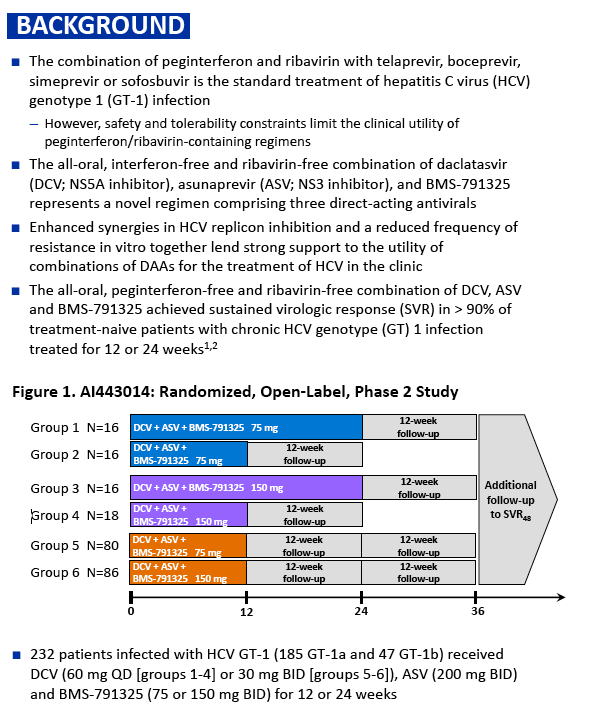
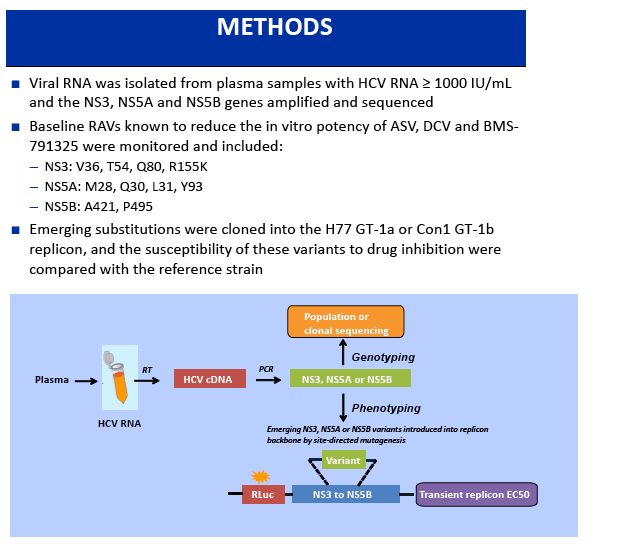
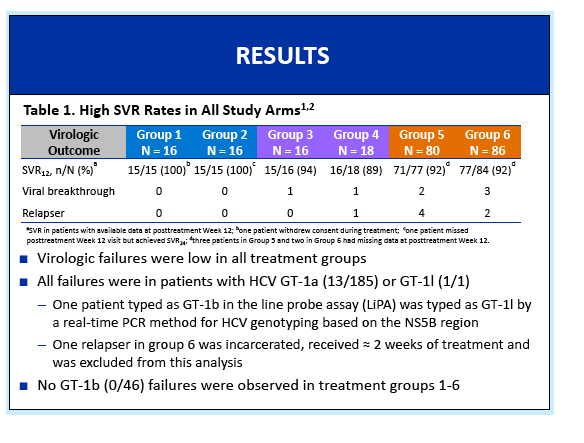
Phase 3 Unity Study - A Study of an Investigational Treatment Regimen of Daclatasvir (DCV) + Asunaprevir (ASV) + BMS-791325 in a Fixed Dose Combination (the Triple Regimen) for 12 Weeks for the Treatment of Chronic Hepatitis C Virus (HCV) Genotype 1 Infection in Non-cirrhotic Subjects
Phase 3 Unity Study - A Study of an Investigational Treatment Regimen of Daclatasvir(DCV)+Asunaprevir(ASV)+BMS-791325 in a Fixed Dose Combination(the Triple Regimen)With or Without Ribavirin(RBV)for 12 Weeks for the Treatment of Chronic Hepatitis C Virus(HCV)Genotype 1 Infection in Subjects With Compensated Cirrhosis
Phase III HIV/HCV Co-Infection Daclatasvir (DCV)+ Sofosbuvir (SOF).......in Treatment-naïve and Treatment-experienced Chronic Hepatitis C (Genotype 1, 2, 3, 4, 5, or 6)
Phase III Daclatasvir + Sofosbuvir in Cirrhotic Subjects and Subjects Post-liver Transplant.......A Phase 3 Evaluation of Daclatasvir and Sofosbuvir in Genotype 1-6 Chronic Hepatitis C Infection Subjects With Cirrhosis Who May Require Future Liver Transplant and Subjects Post-Liver Transplant......This trial is open to patients with cirrhosis due to chronic HCV, and to patients who have already received a liver transplant for chronic HCV. All subjects will be treated with Daclatasvir and Sofosbuvir for 12 weeks. Under certain conditions, the treatment duration may be extended for cirrhotic subjects. The study will test how well this combination of investigational drugs works to treat chronic HCV.
Phase III Daclatasvir and Sofosbuvir for Genotype 3 Chronic HCV
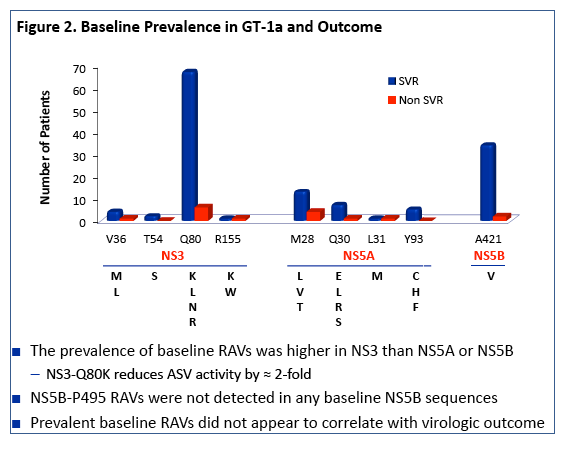
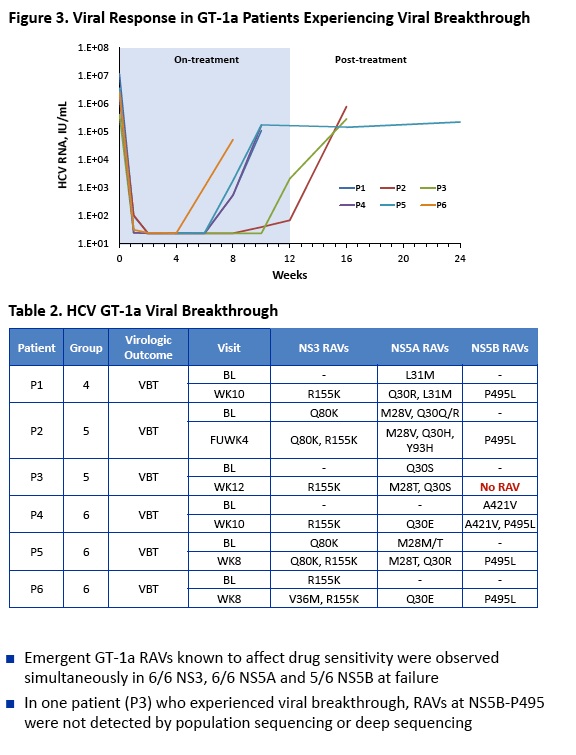
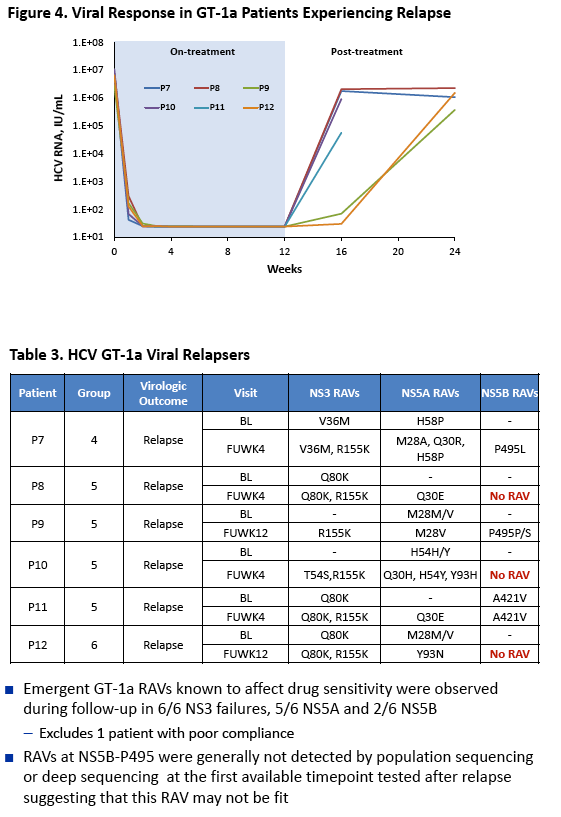
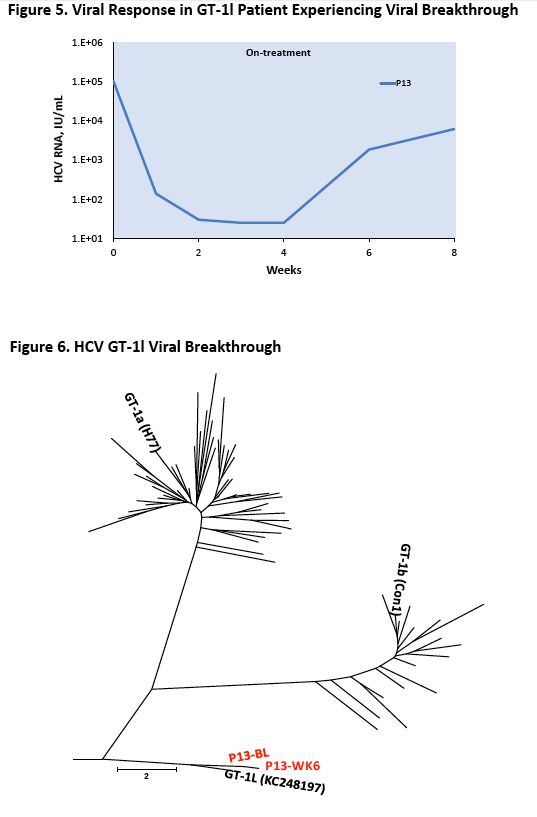
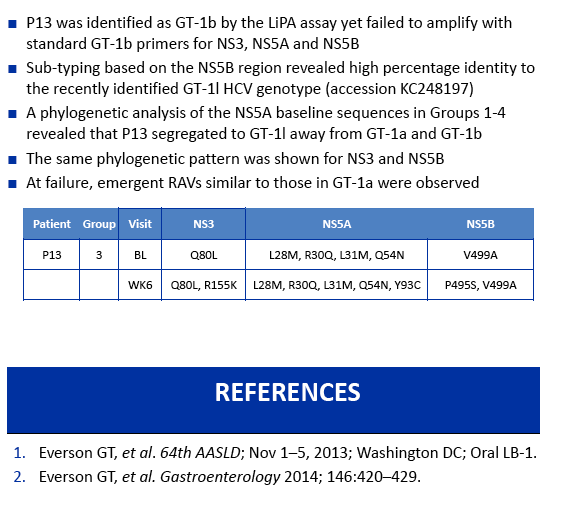
|
| |
|
 |
 |
|
|