 |
 |
 |
| |
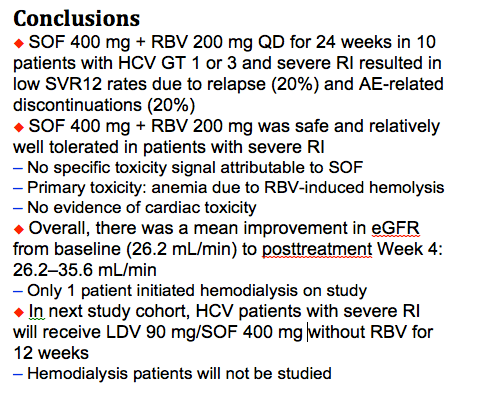
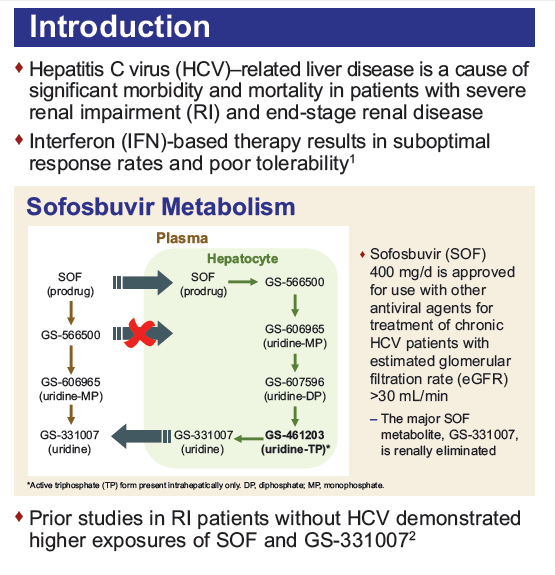
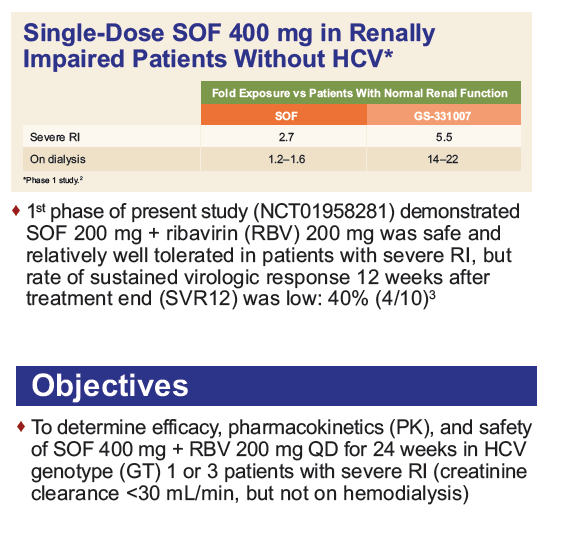
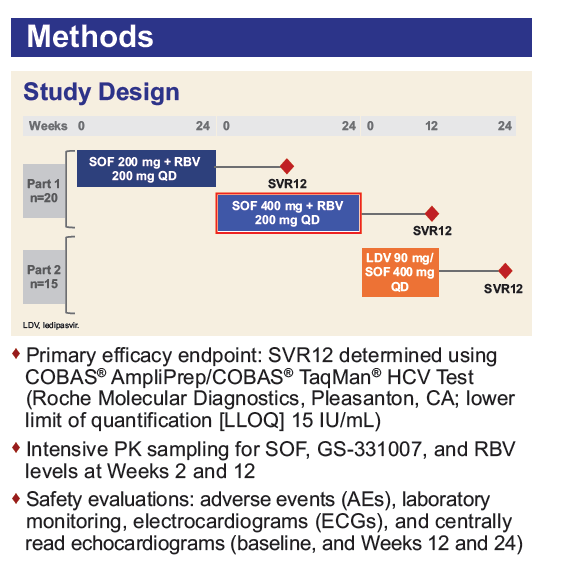
Safety and Efficacy of Treatment With Daily Sofosbuvir 400 mg + Ribavirin 200 mg for 24 Weeks in Genotype 1 or 3 HCV-Infected Patients With Severe Renal Impairment
|
| |
| |
Reported by Jules Levin
Paul Martin,1 Edward Gane,2 Grisell Ortiz-Lasanta,3 Lin Liu,4 Karim Sajwani,4 Brian Kirby,4 Luisa M. Stamm,4 Diana M. Brainard,4 John G. McHutchison,4 Eric Lawitz,5 Stuart Gordon,6 Richard Robson7
1University of Miami, FL; 2Auckland City Hospital, Auckland, New Zealand; 3Fundacion de Investigacion, Rio Piedras, PR; 4Gilead Sciences, Inc., Foster City, CA; 5The Texas Liver Institute, UT Health Science Center, San Antonio, TX; 6Henry Ford Health System, Detroit, MI; 7Christchurch Clinical Studies Trust Ltd, Christchurch, New Zealand
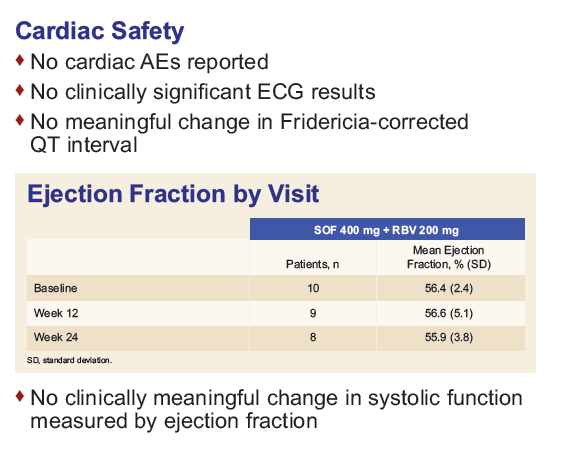
References
1. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. hcvguidelines.org; 2. Cornpropst M, et al. EASL 2012, poster 1101; 3. Gane E, et al. AASLD 2014, poster 966; 4. Sulkowski MS, et al. JAMA 2014;312:353-61.
|
| |
|
 |
 |
|
|