 |
 |
 |
| |
Enrollment and preliminary follow-up of injecting drug users receiving pre-exposure prophylaxis in Bangkok
|
| |
| |
Reported by Jules Levin
CROI 2015 Feb 23-26, Seattle, WA
Michael Martin1, Philip Mock1, Marcel Curlin1, Suphak Vanichseni2, Pravan Suntharasamai2, Udomsak Sangkum2, Kachit Choopanya2, Manoj Leethochawalit3, Sithisat Chiamwongpaet3, Somyot Kittimunkong4
1 US Centers for Disease Control and Prevention (CDC), Nonthaburi, Nonthaburi, Thailand;2 Bangkok Tenofovir Study Group, Bangkok, Thailand;3 Bangkok Metropolitan Administration, Bangkok, Thailand;4 Ministry of Public Health, Nonthaburi, Thailand
"The Bangkok Tenofovir Study (BTS) was a randomized, double-blind,
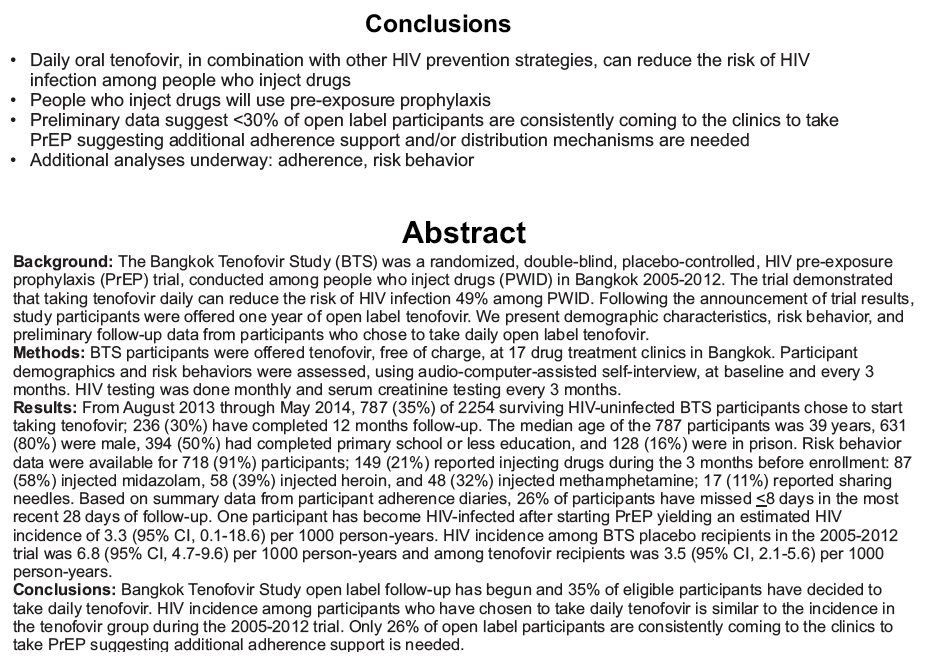
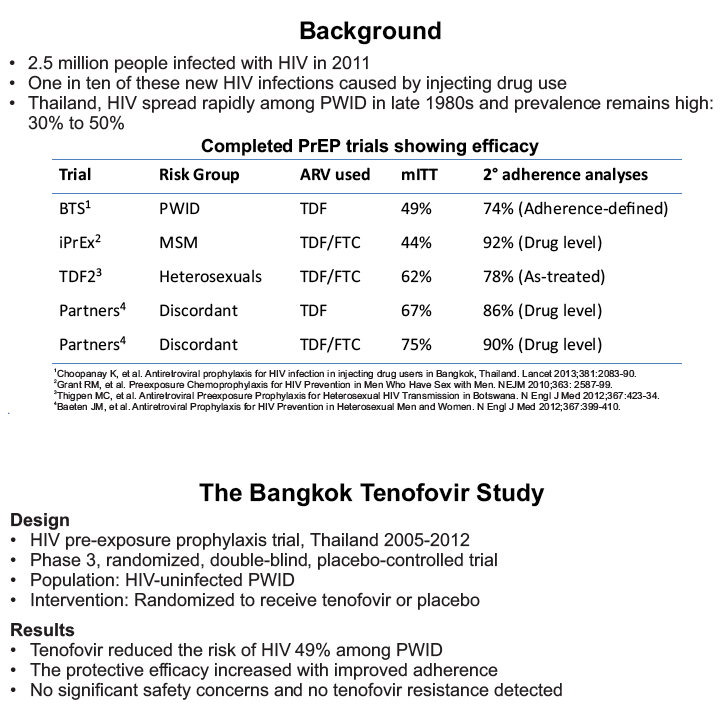
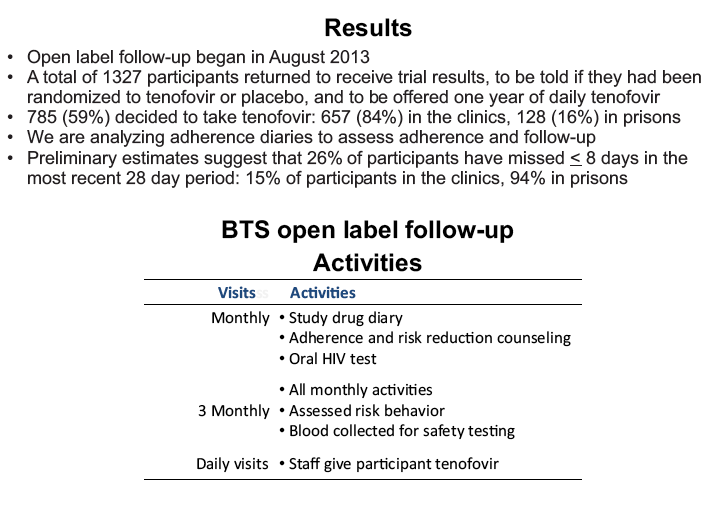
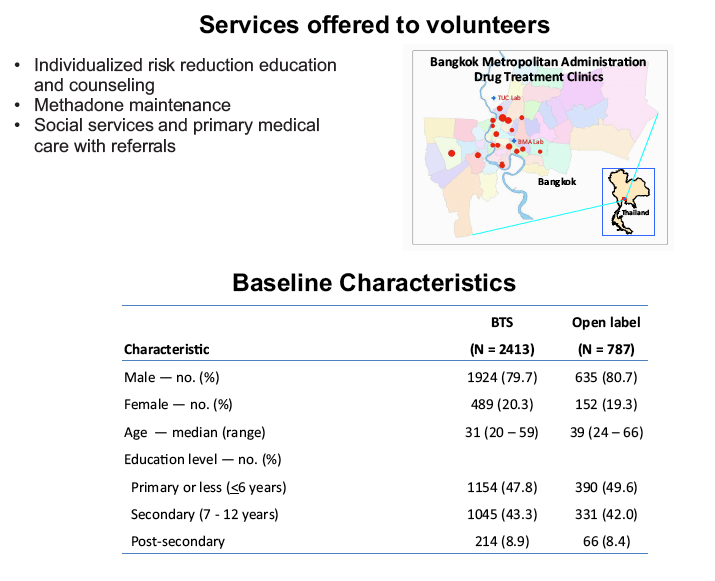
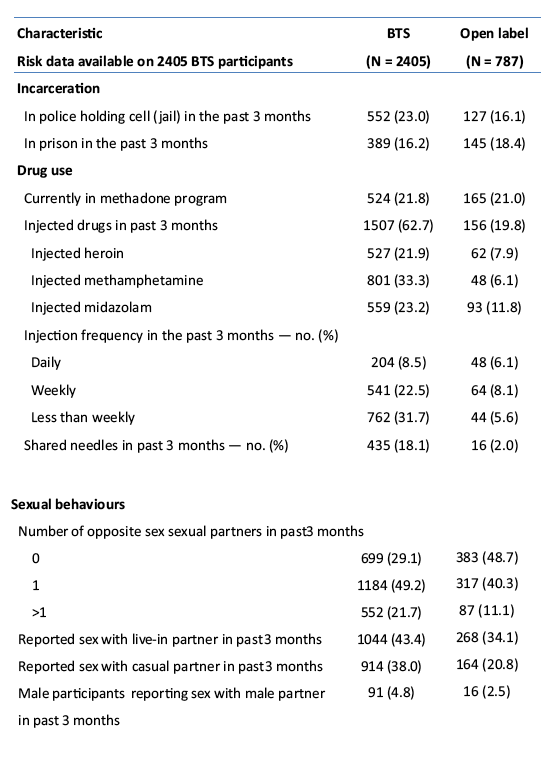
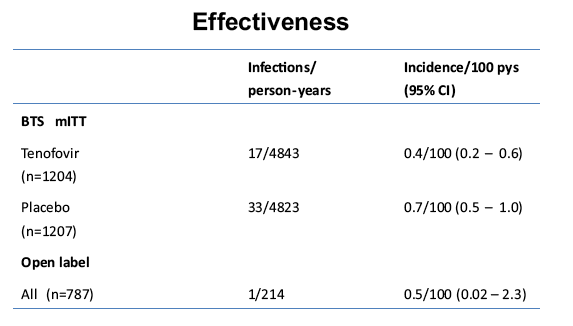
placebo-controlled, HIV pre-exposure prophylaxis (PrEP) trial, conducted among people who inject drugs (PWID) in Bangkok 2005-2012. The trial demonstrated that taking tenofovir daily can reduce the risk of HIV infection 49% among PWID. Following the announcement of trial results, study participants were offered one year of open label tenofovir......BTS participants were offered tenofovir, free of charge, at 17 drug treatment clinics in Bangkok......From August 2013 through May 2014, 787 (35%) of 2254 surviving HIV-uninfected BTS participants chose to start taking tenofovir; 236 (30%) have completed 12 months follow-up..... One participant has become HIV-infected after starting PrEP yielding an estimated HIV.....incidence of 3.3 (95% CI, 0.1-18.6) per 1000 person-years. HIV incidence among BTS placebo recipients in the 2005-2012 trial was 6.8 (95% CI, 4.7-9.6) per 1000 person-years and among tenofovir recipients was 3.5 (95% CI, 2.1-5.6) per 1000 person-years. Conclusions: Bangkok Tenofovir Study open label follow-up has begun and 35% of eligible participants have decided to take daily tenofovir. HIV incidence among participants who have chosen to take daily tenofovir is similar to the incidence in the tenofovir group during the 2005-2012 trial. Only 26% of open label participants are consistently coming to the clinics to take PrEP suggesting additional adherence support is needed."
|
| |
|
 |
 |
|
|