 |
 |
 |
| |
Impact of TDF + PI/r on Renal Function in
Sub-Saharan Africa (2LADY/ANRS 12169)
|
| |
| |
...... program abstract - 'The aim was to determine if TDF+PI/r use [LPV/r & DRV/r] was associated with changes in renal function in HIV patients starting a second line ART in comparison with a regimen without TDF (abacavir (ABC) + didanosine (ddI) + LPV/r) in three African countries (Cameroun, Senegal and Burkina Faso)........ Results: Out of 454 randomized patients, 443 were included in this analysis. Mean age was 39 years (SD: 9.7) and 72% were women. Thirty-nine patients (9%) had hypertension and 2 (0.4%) diabetes. Median follow-up was 16 months.
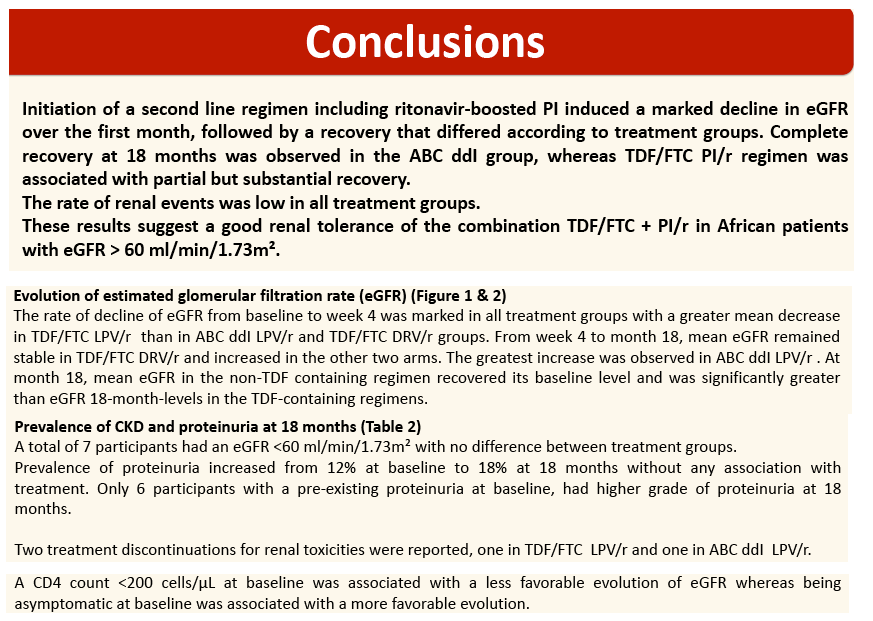
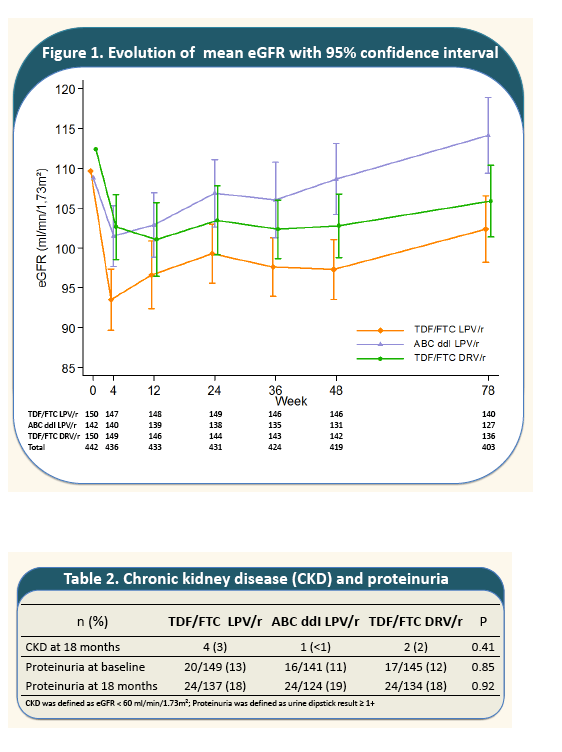
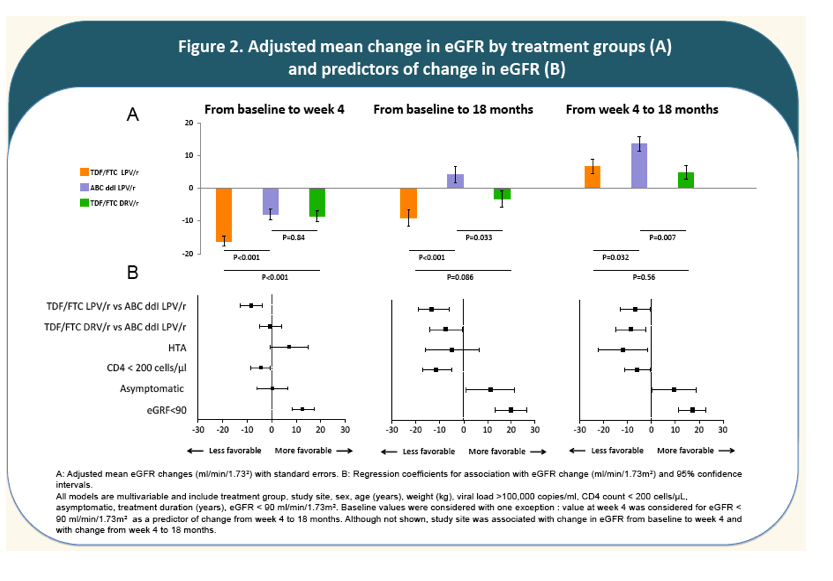
The rate of decline of eGFR from baseline to week 4 was marked in all treatment groups with a greater mean decrease in TDF+FTC+LPV/r arm (-16.6 ml/min/1.73m2 ) than in ABC+DDI+LPV/r arm (-8.2; P=10-3); and TDF+FTC+DRV/r arm (-9.6; P=10-3).
From week 4 to month 18, mean eGFR remained stable in TDF+FTC+DRV/r and increased in the other two arms. The greatest increase was observed in ABC+DDI+LPV/r (+10.4). At month 18, mean eGFR in the non-TDF containing regimen (112.3) recovered its baseline level and was significantly greater than eGFR 18-month-levels in the TDF-containing regimens (with LPV/r; 100.8; P=5.10-3; with DRV/r; 104.9; P=0.02). Incidence of CKD was 5%; 5% and 4% person-year in arms A, B and C, respectively and did not differ by treatment arms (P=0.78).
Conclusions: Randomization to TDF+lP/r was associated with a mild non progressive decrease of eGFR after 18 months in patients with eGFR>60ml/ml/1,73m2 at baseline. These results suggest a good renal tolerance of the second line associating TDF+FTC+IP/r in patients in sub-Saharan Africa."
Reported by Jules Levin
CROI 2015 Feb 23-26, Seattle, WA
Arsene Hema1, Amandine Cournil2, Laura Ciaffi3, Sabrina Eymard-Duvernay2, Assane Diouf4, Nestor Manga5, Vincent Le Moing2, Jacques Reynes2, Sinata Koulla-Shiro6, Eric Delaporte2
1CHU Souro Sanou, Bobo Dioulasso, Burkina Faso ; 2UMI 233 - IRD / U1175 - INSERM / Montpellier University, Montpellier, France; 3ANRS Research Center, Yaounde, Cameroon; 4CRCF, Dakar, Senegal; 5Yaounde Military Hospital, Yaounde, Cameroon; 6FMSB/University Yaounde 1, Yaounde, Cameroon
- A CD4 count <200 cells/μL at baseline was associated with a less favorable evolution of eGFR whereas being asymptomatic at baseline was associated with a more favorable evolution
- A total of 7 participants had an eGFR <60 ml/min/1.73m· with no difference between treatment groups.
- Prevalence of proteinuria increased from 12% at baseline to 18% at 18 months without any association with treatment. Only 6 participants with a pre-existing proteinuria at baseline, had higher grade of proteinuria at 18 months.
Two treatment discontinuations for renal toxicities were reported, one in TDF/FTC LPV/r and one in ABC ddI LPV/r.
Comment by Courtney Fletcher in NATAP Pharmacology report on this study:
"A strong genetic susceptibility to CKD has been observed in the sub-Saharan African population. Investigators described the effect of 2nd line ART on renal function in 443 persons from 3 African countries who had failed first line therapy (#793, poster). These participants were randomized to one of 3 regimens: (A), TDF+FTC+LPV/r; (B), ABC+ddI+LPV/r; or (C), TDF+FTC+DRV/r and were followed for 18 months. All 3 groups had a marked decline in eGFR over the first month; the largest decrease in eGFR was in the TDF+FTC+LPV/r recipients. At month 18, mean eGFR in the non-TDF containing regimen of ABC+ddI+LPV/r had recovered to baseline eGFR, and was significantly greater than those in the 2 TDF-containing arms. The incidence of CKD was 5%, 5% and 4% person-year in arms A, B and C, respectively."
Comment in NATAP Kidney Report by Christina Wyatt & Lene Ryom:
During a median of 16 months follow-up, a 5% incidence rate of CKD was observed among 443 HIV-positive women starting 2nd line ART in Cameroon, Senegal or Burkina Faso [Abstract 793]. Overall, the use of tenofovir in combination with boosted lopinavir or darunavir was well tolerated by the large majority of participants based on changes in median eGFR.
program abstract
Background: A strong genetic susceptibility to chronic kidney disease (CKD) has been observed in sub-Saharan African populations. The current WHO-recommended second line antiretroviral treatment (ART) associates a protease inhibitor boosted with ritonavir (PI/r) in combination with Tenofovir Disoproxil Fumarate (TDF) or Zidovudine (AZT) + lamivudine or emtricitabine (FTC). TDF + PI/r has been associated with proximal tubular dysfunction and CKD.
The aim was to determine if TDF+PI/r use was associated with changes in renal function in HIV patients starting a second line ART in comparison with a regimen without TDF (abacavir (ABC) + didanosine (ddI) + LPV/r) in three African countries (Cameroun, Senegal and Burkina Faso).
Methods: HIV-1 positive adults, failing standard first line ART were randomized to either A: TDF+FTC+LPV/r; B: ABC + ddI + LPV/r or C: TDF + FTC + darunavir (DRV) /r and followed until 18 months. Patients with an MDRD-estimated glomerular filtration rate (eGFR) ³ 60 ml/min/1,73m2 were included in this analysis.
We compared levels and changes in eGFR using multivariable linear regressions. First occurrence of CKD (eGFR
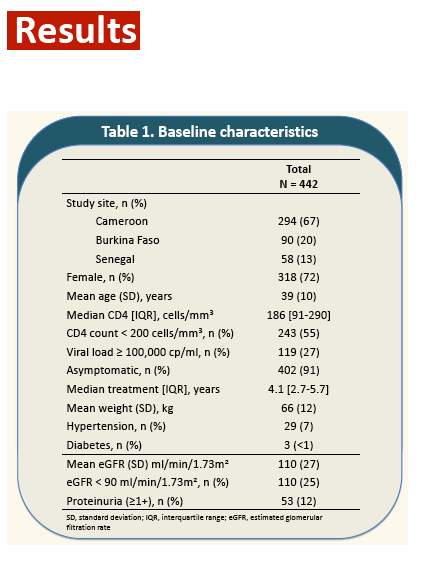
Results: Out of 454 randomized patients, 443 were included in this analysis. Mean age was 39 years (SD: 9.7) and 72% were women. Thirty-nine patients (9%) had hypertension and 2 (0.4%) diabetes. Median follow-up was 16 months.
The rate of decline of eGFR from baseline to week 4 was marked in all treatment groups with a greater mean decrease in TDF+FTC+LPV/r arm (-16.6 ml/min/1.73m2 ) than in ABC+DDI+LPV/r arm
(-8.2; P=10-3); and TDF+FTC+DRV/r arm (-9.6; P=10-3). From week 4 to month 18, mean eGFR remained stable in TDF+FTC+DRV/r and increased in the other two arms. The greatest increase was observed in ABC+DDI+LPV/r (+10.4). At month 18, mean eGFR in the non-TDF containing regimen (112.3) recovered its baseline level and was significantly greater than eGFR 18-month-levels in the TDF-containing regimens (with LPV/r; 100.8; P=5.10-3; with DRV/r; 104.9; P=0.02). Incidence of CKD was 5%; 5% and 4% person-year in arms A, B and C, respectively and did not differ by treatment arms (P=0.78).
Conclusions: Randomization to TDF+IP/r was associated with a mild non progressive decrease of eGFR after 18 months in patients with eGFR>60ml/ml/1,73m2 at baseline. These results suggest a good renal tolerance of the second line associating TDF+FTC+IP/r in patients in sub-Saharan Africa.
|
| |
|
 |
 |
|
|