 |
 |
 |
| |
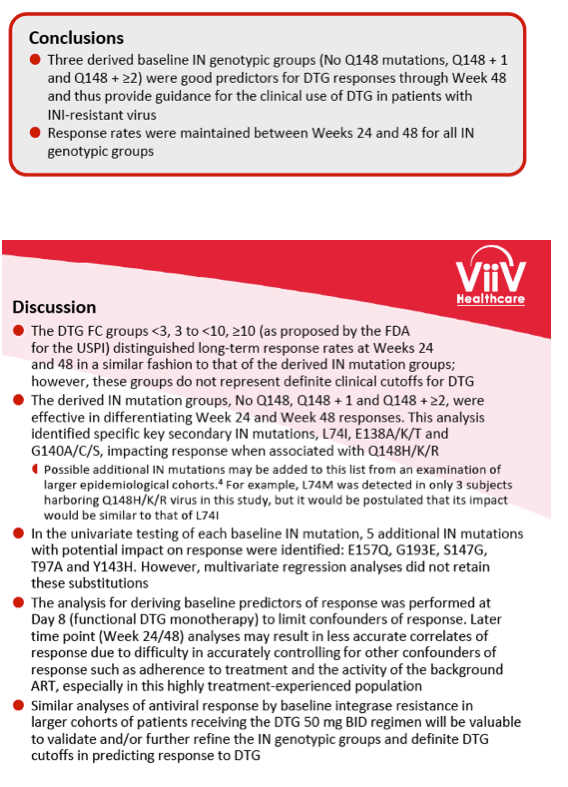
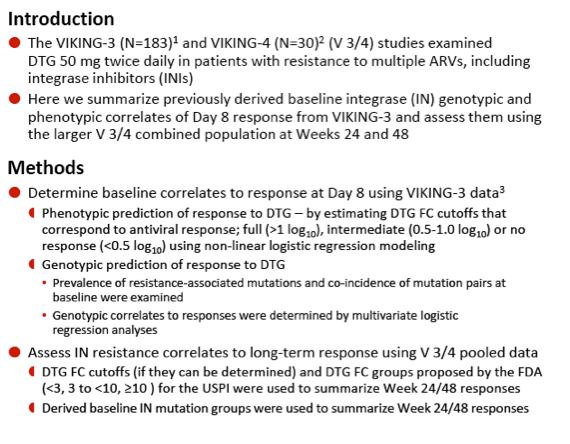
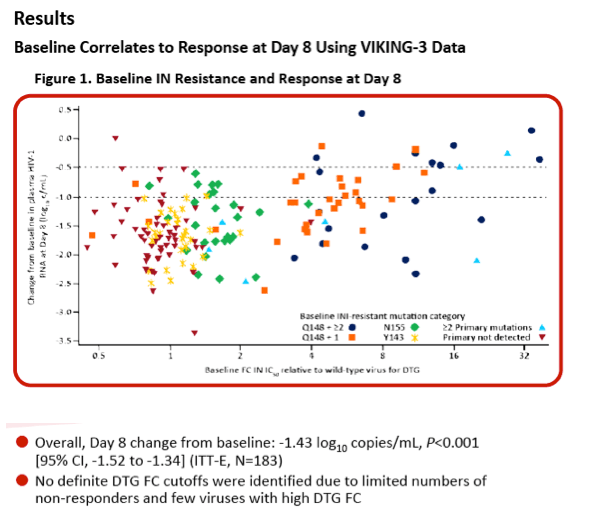
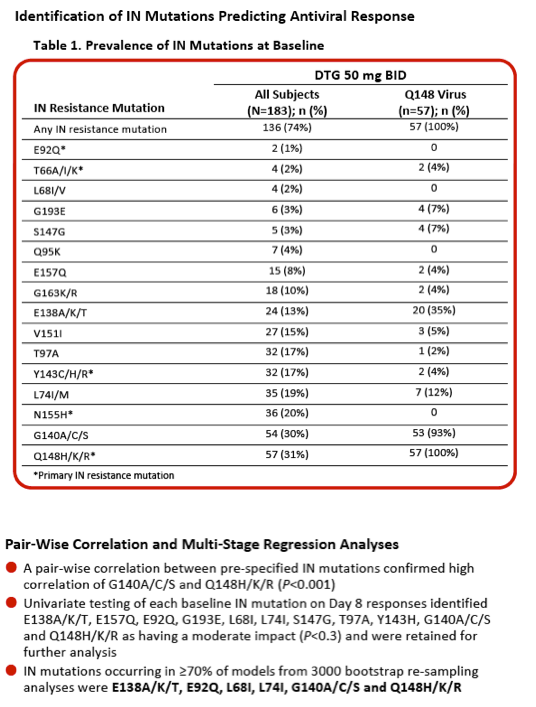
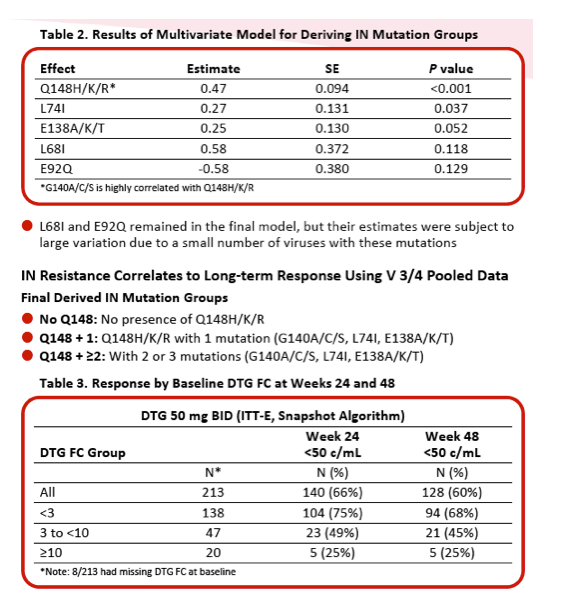
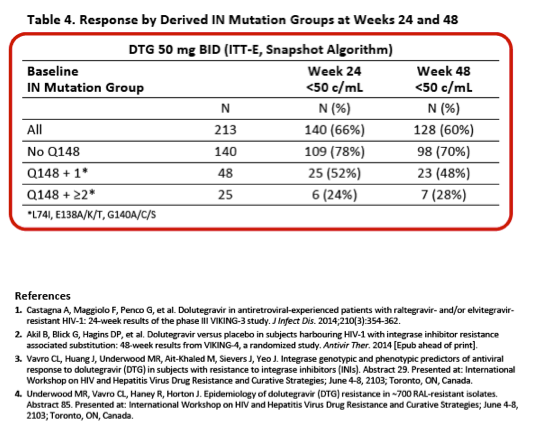
Integrase Resistance Correlates of Response to Dolutegravir (DTG) Through 48 Weeks
|
| |
| |
Reported by Jules Levin
CROI 2015 Feb 23-26, Seattle, WA
Cindy Vavro1, Jenny Huang1, Mounir Ait-Khaled1
1 Infectious Diseases, GlaxoSmithKline, Research Triangle Park, North Carolina, United States
Dolutegravir in Antiretroviral-Experienced Patients With Raltegravir- and/or Elvitegravir-Resistant HIV-1: 24-Week Results of the Phase III VIKING-3 Study........
http://www.natap.org/2014/HIV/071414_01.htm........(Journal of Infectious Diseases Jan 2014, Antonella Castagna)
Activity of Dolutegravir (DTG) 50 mg BID vs Placebo (PBO) Over 7 Days of Functional Monotherapy in Patients Harbouring Raltegravir- and/or Elvitegravir-Resistant Virus: Primary Endpoint Results of the VIKING-4 Study (ING116529) - Antiviral Activity of Dolutegrevir in Subjects With Failure on an Integrase Inhibitor-Based Regimen: Week 24 Phase 3 Results From VIKING-3 (1)
http://www.natap.org/2014/HIV/012214_03.htm
Resistance to HIV Integrase Strand Transfer Inhibitors Among Clinical Specimens in the United States, 2009-2012; Dolutegravir Resistance
Reports.....http://www.natap.org/2014/HIV/012214_03.htm
The Activity of the Integrase Inhibitor Dolutegravir Against HIV-1 Variants Isolated From Raltegravir-Treated
Adults......http://www.natap.org/2012/HIV/110812_01.htm
|
| |
|
 |
 |
|
|