 |
 |
 |
| |
Kidney Dysfunction and Markers of Inflammation
in the Multicenter AIDS Cohort Study
|
| |
| |
Download the PDF here
Reported by Jules Levin
CROI 2015 Feb 23-26, Seattle, WA
Alison Abraham1, Heather McKay1, Joseph Margolick1, Lisa Jacobson1, Annie Darilay2, Michelle Estrella4, Frank Palella5, Robert Bolan6, Charles Rinaldo7
1 Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland, United States;2 MedImmune, AstraZeneca, Gaithersburg, Maryland, United States;4 Johns Hopkins University School of Medicine, Baltimore, Maryland, United States;5 Northwestern University, Feinberg School of Medicine, Chicago, Illinois, United States;6 Los Angeles LGBT Center, Los Angeles, California, United States;7 University of Pittsburgh, Pittsburgh, Pennsylvania, United States
program abstract
Background: HIV-associated chronic immune activation and inflammation may contribute to increased risk for kidney disease among HIV-infected (HIV+) individuals, even after viral suppression. We examined associations between inflammatory processes and kidney function in treated and virally suppressed HIV+ and HIV- men from the Multicenter AIDS Cohort Study.
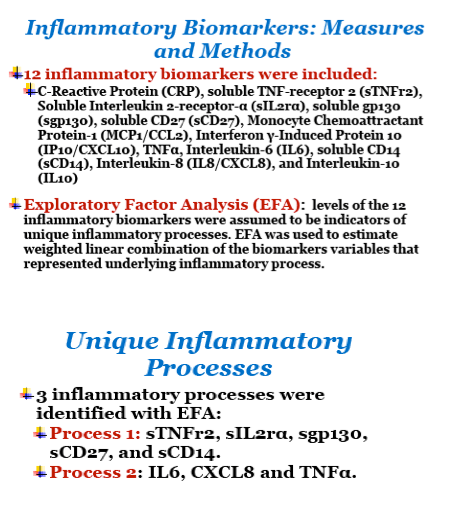
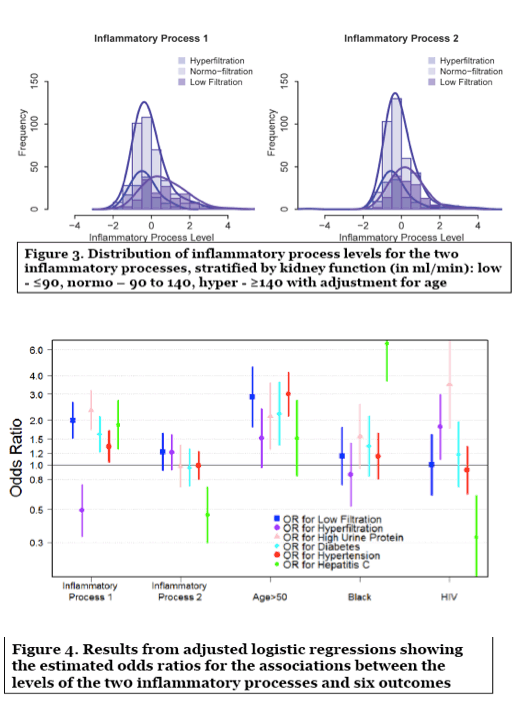
Methods: Glomerular filtration rate (GFR) was directly measured using decay of plasma iohexol concentration. Twelve markers of inflammation were measured from stored blood samples (C-Reactive Protein, Soluble TNF-receptor 2, Soluble Interleukin (IL) 2-receptor-α, Soluble GP130, Soluble CD27, Monocyte Chemoattractant Protein-1, Interferon γ-Induced Protein 10, TNF-α, IL-6, Soluble CD14, IL-8, IL-10). Exploratory factor analysis (EFA) was used to identify underlying inflammatory processes, and factors were validated in an independent dataset. The validated factor scores were used in adjusted logistic regression analyses to evaluate their associations with kidney outcomes: reduced GFR (GFR≤90 ml/min/1.73m2); hyperfiltration (GFR >140 ml/min/1.73m2 - 1 ml/min/1.73m2 for each year over age 40); and urine protein:creatinine ratio (uPCr) category ( ≤100, 100-200, >200 mg/g).
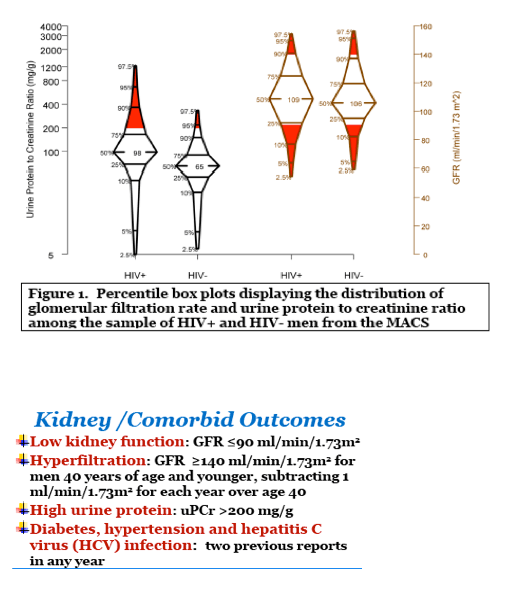
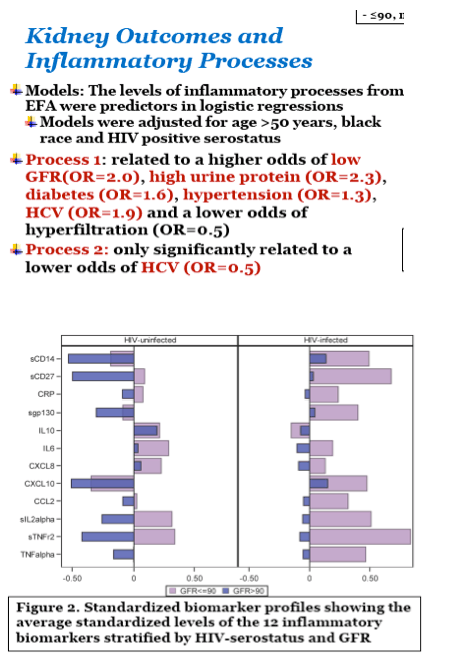
Results: 434 HIV+ men, all on antiretroviral therapy and 80% virally suppressed, and 200 HIV- men had available GFR determinations and inflammatory biomarker levels. HIV+ men had higher levels of cystatin C (median: 0.79 versus 0.75 mg/dl) and uPCr (median: 98 versus 66 mg/g). From the EFA, three factors were retained that accounted for 60% of the total variance in inflammatory marker levels; only the first two factors could be replicated in a validation set. Factor 1 (dominated by markers: sTNF receptor 2, sIL 2-receptor-α, sGP130, sCD27, and sCD14) scores (shown in the Figure) were significantly (p2 (OR=2.0), a greater odds of uPCr >200 mg/g (OR=2.2), and a lower odds of hyperfiltration (OR=0.5), as well as with a history of diabetes (OR=1.6) and a history of hypertension (OR=1.3). Levels of all of these markers except GP130 were significantly higher in HIV+ men. Factor 2 (dominated by markers: IL-6, IL-8 and TNF-α) was not significantly associated with any kidney outcomes.
Conclusions: Higher circulating levels of immune activation markers among treated HIV+ individuals, despite virologic suppression, may partially explain their higher burden of kidney dysfunction compared to HIV- persons.
Process 1 was associated with both lower GFR and higher urine protein, and the markers in that process are, generally speaking, markers of immune activation. Process 2 was associated with hyperfiltration, a sign of early kidney damage, only among HIV-infected. There is no way to tell from this study what is driving what as low filtration could lead to accumulation of markers in the blood. We can posit that HIV-mediated destruction of gut mucosa leads to translocated microbial products and chronic activation of monocytes as well as a hypercoagulable state (even in those who are virally suppressed). The combined effect of systemic inflammation and excess clotting on tissue function leads to end-organ disease including, potentially, kidney dysfunction. ThereÕs certainly some evidence that this model could be one reason why HIV-infected tend to have a higher burden of multimorbidity - Steve Deeks has a nice paper on this in Immunity Perspective (2013). There arenÕt many longitudinal studies looking comprehensively at large panel of markers and incident kidney outcomes, which would be the best way to assess what is driving what. However, the MACS has the capacity to do that using data from our second iohexol GFR measurement, with augmentation from estimated GFRs. So stay tuned.
|
| |
|
 |
 |
|
|