 |
 |
 |
| |
Patient Reported Outcomes Over 48 Weeks in a
Randomized, Open-Label Trial of Patients With
HIV Switching to Coformulated Elvitegravir, Cobicistat, Emtricitabine, and Tenofovir Alafenamide (E/C/F/TAF)
|
| |
| |
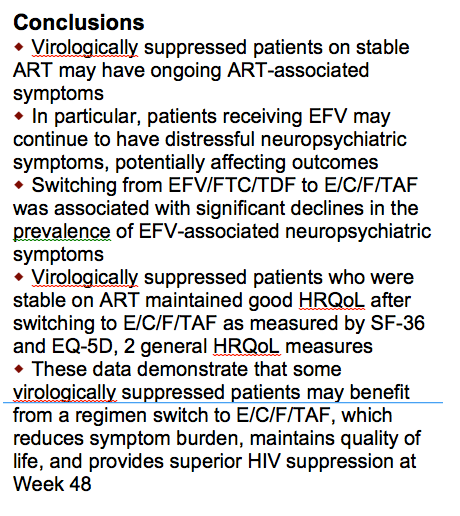
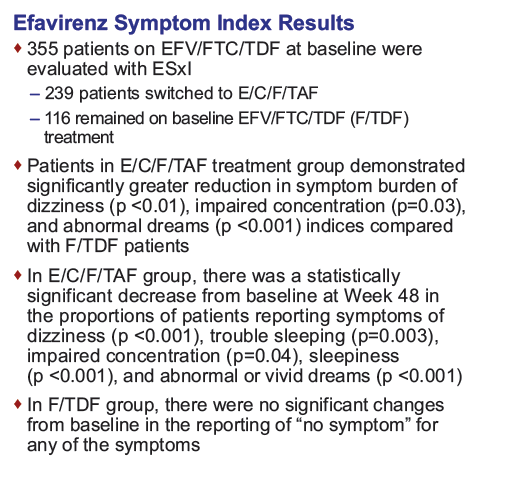
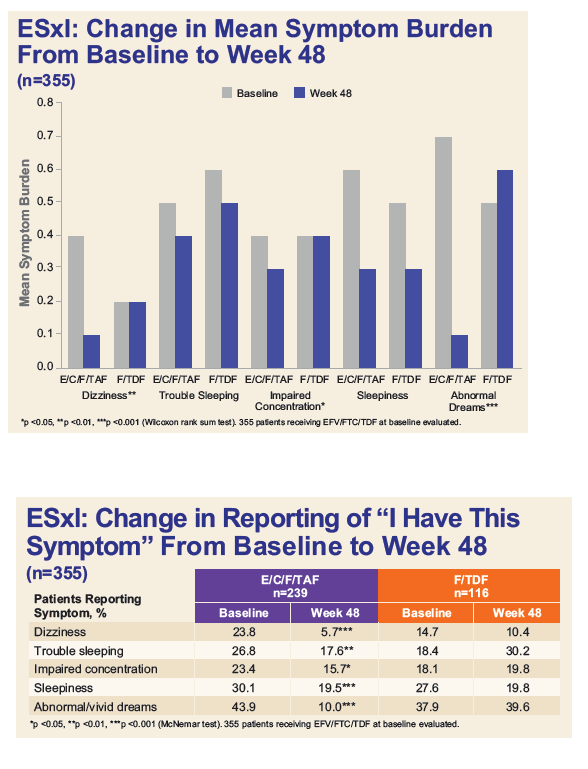
....Switching off efavirenz from EFV/FTC/TDF to E/C/F/TAF was associated with significant declines in the prevalence of EFV-associated neuropsychiatric symptoms
Reported By Jules Levin
EACS 2015 Oct 21-24 Barcelona, Spain
Thomas Lutz,1 Paul Benson,2 Jean-Christophe Goffard,3 Richard Haubrich,4 David Budd4
1Infektiologikum, Frankfurt am Main, Germany; 2Be Well Medical Center, Berkeley, Michigan, USA; 3H˘pital Erasme, Brussels, Belgium; 4Gilead Sciences, Inc., Foster City, California, USA

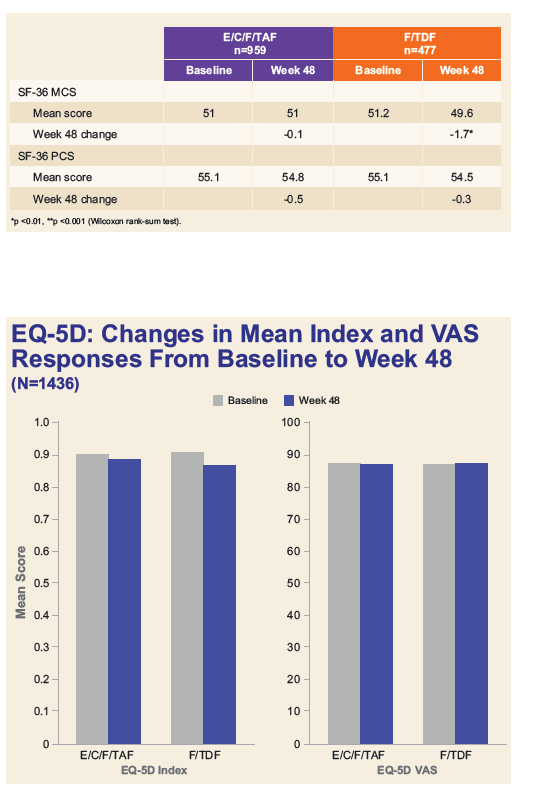
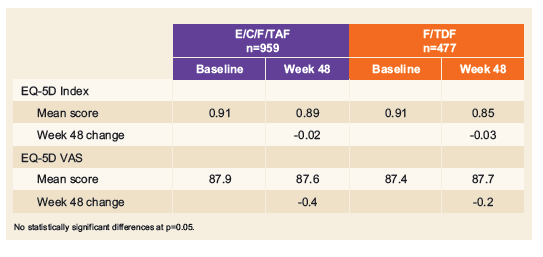
References
1. Al-Dakkak I, et al. AIDS Care 2013;25(4):400-14; 2. Rosenblum M, et al. PLoS One 2009;4(9):e7196; 3. Sweet D, et al. IAS 2014. Abstract THPE421; 4. Edelman EJ, et al. AIDS Behav 2011;15(4):853-61;
5. Mills A, et al. IAS 2015. Abstract TUAB0102; 6. Sustiva [package insert] Princeton, NJ: Bristol-Myers Squibb Pharma Company; 2015; 7. The EuroQol Group. Health Policy 1990;16(3):199-208; 8. Hsiung PC,
et al. Qual Life Res 2005;14(1):141-50.
|
| |
|
 |
 |
|
|