| |
Federal Healthcare Solution Needed - Specialty drugs cost increasing for cancers, diabetes, heart disease, autoimmune diseases which are not curable
|
| |
| |
Jules Levin, NATAP
New treatment for serious diseases are being developed for diabetes, cancers, HCV, heart disease, autoimmune diseases. Costs for these treatments are bending the healthcare system so a federal response is needed, not to deny access but to facilitate access. The job of the federal govt is to safeguard the health of its citizens, instead it facilitates State Medicaids in imposing severe restrictions in denying access by patients to new potent HCV medications, which are not treatments but "cures", yes with 12 weeks tolerable short-term therapy these treatments can cure HCV and the rates of SVR/cure rates are up to 100% with more effective treatments coming soon, in development. The imposition of these restrictions are in the face of the prices having recently been cut by 50% by the drug manufacturers to medicaid & the VA. This is a unique situation that is often not understood nor explained to people. HIV therapy is lifetime, about $15,000 a year for the "cocktail" multi-drug treatment, but on top of that is the cost of lifetime care, together these costs add up to $500,000 for 1 HIV+ patient over the course of 25 years, this is the cost to the healthcare system & to the federal govt. The federal govt spends $22 billion a year on domestic HIV, $4.5 billion on medicare and $5 billion on medicaid, every year ! With the 50% discount the HCV drug manufacturers just provided to state medicaids and to the VA the one-time cost is $46,000, but it is a one-time cost & but the cost is all upfront, not spread over 30 years. That is the problem, the US healthcare system is not designed to pay all this cost upfront in one fiscal quarter, that is why the public & private insurance payers are complaining, because that cost must be paid upfront in one fiscal quarter, so it goes on their books as a cost for that quarter & hits their budget that year, and their budgets are annual only. That is why we need a new model to fund healthcare, see just below the link to an article written by a famous healthcare economist where he outlines a potential solution. But there are numerous solutions that could be implemented. But the federal govt, the White house, the Adminstration & Congress are not discussing this & the public is not either. We need a public debate about this. And these new expensive cancer, diabetes & heart disease drugs are more expensive than the HCV drugs & are NOT cures, they are for ongoing long term or lifetime therapy. That is what distinguishes HCV from these other diseases & makes it that much more compelling that HCV needs to be addressed, the situation needs to be fixed. In the meantime, since the HCV drug manufacturers have provide a 50% discount it is time to eliminate the medicaid restrictions & permit unfettered access for patients to the new treatments. We could eliminate HCV in the US if we had unfettered access to treatment. We would have to design "test & treat" programs which would have to include linkage to care, training for doctors, and support services for patients & clinics. But the first order of business is to eliminate the restrictions to the drugs. I met a patient yesterday in NYS who was denied HCV treatment by his new Obamacare health plan and their doctor had to put the person on psychiatric therapy because they became so depressed and distraught because he was denied treatment. On top of that this patient has started experiencing fatigue. This patient has stage 2 disease and the restrictions allow only to treat patients with stage 3/4 disease, how absurd because the tests that identify a patient's stage of disease cannot reliably distinguish between stage 2 and stage 3, so with that in mind this patient was denied treatment. If this patient progresses to cirrhosis before being allowed access to treatment, their risk for liver cancer (HCC) & death increases and if it is then treated & cured they will have to do an MRI every 6 months for the remainder of his life to see if HCC develops, that is a cost of $4000 a year, multiply that by 20 years, he is 32 years old now, and the total cost is $80,000, much higher than the cost of the HCV drug treatment. This is the situation now in HCV, the state & federal govt must fix this NOW!
Expensive Cancer, Heart Disease, Diabetes Drugs over the long run more expensive than HCV - (03/16/15)
Abilify Costs 3x more to Medicaid - Abilify costs Missouri medicaid $75 mill last year......Gilead Sciences' Sovaldi - which treats the liver disease and is more effective with fewer side effects than earlier medications - to 311 patients at a cost of $26 million in the fiscal year that ended June 30, 2014.......The Department of Social Services in January announced that it negotiated a lower cost and rebate for a newly introduced competitor to Sovaldi, AbbVie's Viekera Pak, which would be the preferred drug to treat the liver disease. The department estimated the change would save the state about $4.2 million in projected costs.
http://www.columbiamissourian.com/a/187553/specialty-generic-drug-costs-drive-medicaid-costs-up/
New Models for Healthcare Funding Needed.....
http://www.natap.org/2014/HCV/080614_02.htm
--------------------------------
Specialty drugs are driving up the U.S. medication spend - indeed a prime revenue opp
http://medcitynews.com/2015/04/specialty-drugs-driving-u-s-medication-spend/
Need proof that orphan indication's the way to go? The demand for specialty medications - and their premium pricing - is driving up the total American spend on medication, a new study says.
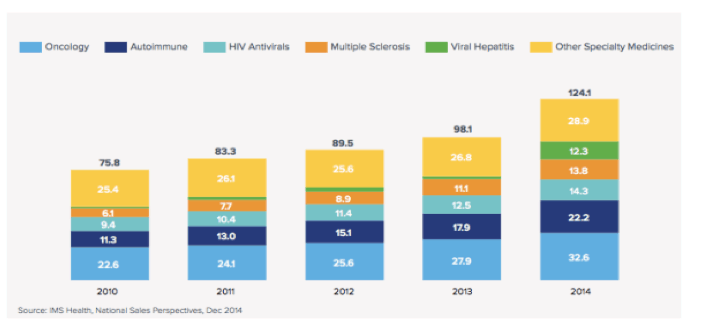
A revenue darling for most budding biotechs, specialty drugs acccounted for $124.1 billion of medication dollars spent, out of a total of $373.9 billion overall, according to a new study from the IMS Institute for Healthcare Informatics. Cancer, hepatitis C and autoimmune disease are the main drivers, with orphan indications filling in the gaps of the specialty medicine super-spend.
Of course, medication costs rose across the board in 2014. The total spending on U.S. medications rose 10.3 percent in 2014, And the total dollars spent on medicine rose 13.1 percent that year, to $373.9 billion - up from a 3.2 percent increase in 2013.
There's a growing number of innovative treatment options out there - but these specialized therapies come with a high price tag. Patent expiries have slowed spending growth, but branded medications keep increasing their price tags. Of course, this is expected to level out next year, IMS says.
Specialty med costs grew 26.5 percent in 2014 alone. While five years ago they accounted for 23 percent of the total medication spend, it's increased to a third. New drugs accounted for $20.3 billion in cost growth for 2014, with more than half - $11.3 billion - from the four new Hep C treatments, including Sovaldi, that made it big on the market. IMS says:
The drug R&D pipeline has shifted to specialty medicines over the past decade and 42% of the late stage pipeline is now specialty, up from 33% ten years ago. Ten Breakthrough Therapies launched in 2014 after the 2012 FDASIA Act granted new approval authorities to the FDA.
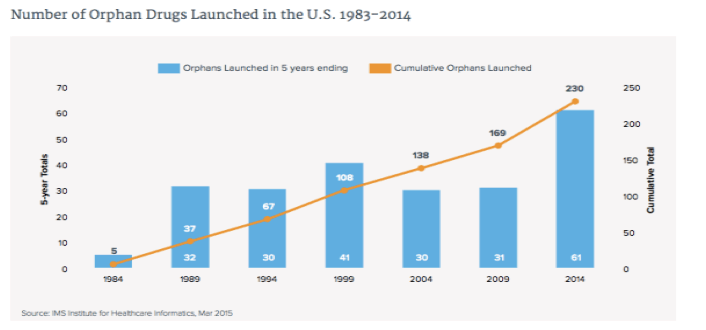
The FDA has a range of new incentive programs including efforts to encourage drug development for antibacterial resistance. In addition the number of orphan drugs launched peaked again with 18 in 2014 and 61 in the last five years.
Cancer remains the most common orphan category, and increasingly very rare "ultra-orphan" drugs, for populations fewer than 10,000, are being developed.
Here's how that breakdown looks, according to specialty medication segment:
Some notable points:
· Spending on specialty medicines increased $54 billion in the last five years, which is 73% of overall medicine spending growth in that period.
· Autoimmune disease, hepatitis C and oncology accounted for $34.7 billion of that increased spending. Hepatitis C in particular was king - more than 161,000 patients started treatment for the disease in 2014.
· The multiple sclerosis spend grew 24.4 percent, thanks to new drugs that have new mechanisms of action and more convenient dosing
· Some $20.2 billion of the drug spend came from newly approved, branded drugs - and 78 percent of those dollars came from specialty drugs.
The report found that more new medications were launched in 2014 than any year since 2001, and included 23 breakthrough therapies and orphan drugs. IMS says:
Aided by expedited approval pathways and FDA incentive programs for rare and infectious diseases, a robust late-phase pipeline with more than 530 distinct research programs is expected to maintain the high number of launches seen in the past five years into the future.
Biosimilars will change up the medication landscape soon, though - these cost increases aren't expected to be sustained.
|
|
| |
| |
|
|
|