| |
FDA approved changes to the Olysio (simeprevir) package insert to include two new Warnings and Precautions;
|
| |
| |
1. serious symptomatic bradycardia when co-administered with sofosbuvir and amiodarone
2. hepatic decompensation and hepatic failure.
Additional changes to the Indication and Usage, Dosage and Administration, Adverse Reactions, Drug Interactions, Use in Specific Populations and Pharmacokinetic sections of the label were made based on these Warnings and Precautions and are summarized below.
Summary of new WARNINGS AND PRECAUTIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serious Symptomatic Bradycardia When Co-administered with Sofosbuvir and Amiodarone
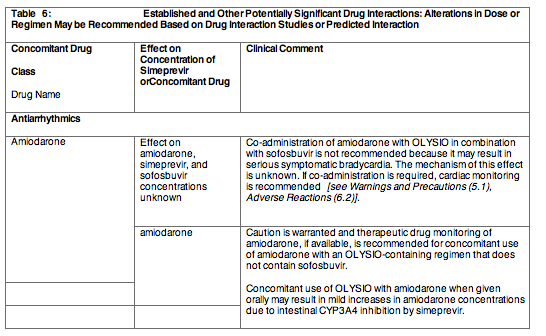
Postmarketing cases of symptomatic bradycardia and cases requiring pacemaker intervention have been reported when amiodarone is co administered with sofosbuvir in combination with another HCV direct acting antiviral, including OLYSIO. A fatal cardiac arrest was reported in a patient receiving a sofosbuvir-containing regimen (ledipasvir/sofosbuvir). Bradycardia has generally occurred within hours to days, but cases have been observed up to 2 weeks after initiating HCV treatment. Patients also taking beta blockers, or those with underlying cardiac comorbidities and/or advanced liver disease may be at increased risk for symptomatic bradycardia with co administration of amiodarone. Bradycardia generally resolved after discontinuation of HCV treatment. The mechanism for this bradycardia effect is unknown.
Co administration of amiodarone with OLYSIO in combination with sofosbuvir is not recommended. For patients taking amiodarone who have no other alternative treatment options, and who will be co administered OLYSIO and sofosbuvir:
· Counsel patients about the risk of serious symptomatic bradycardia
· Cardiac monitoring in an in-patient setting for the first 48 hours of co administration is recommended, after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.
Patients who are taking sofosbuvir in combination with OLYSIO who need to start amiodarone therapy due to no other alternative treatment options should undergo similar cardiac monitoring as outlined above.
Due to amiodarone's long elimination half-life, patients discontinuing amiodarone just prior to starting sofosbuvir in combination with OLYSIO should also undergo similar cardiac monitoring as outlined above.
Patients who develop signs or symptoms of bradycardia should seek medical evaluation immediately. Symptoms may include near-fainting or fainting, dizziness or lightheadedness, malaise, weakness, excessive tiredness, shortness of breath, chest pain, confusion or memory problems [see Adverse Reactions (6.2) and Drug Interactions (7.3)].
5.2 Hepatic Decompensation and Hepatic Failure
Hepatic decompensation and hepatic failure, including fatal cases, have been reported postmarketing in patients treated with OLYSIO in combination with peginterferon alfa and ribavirin or in combination with sofosbuvir. Most cases were reported in patients with advanced and/or decompensated cirrhosis who are at increased risk for hepatic decompensation or hepatic failure. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between treatment with OLYSIO and these events has not been established [see Adverse Reactions (6.2)].
OLYSIO is not recommended for patients with moderate or severe hepatic impairment (Child-Pugh Class B or C) [see Dosage and Administration (2.5) and Use in Specific Populations (8.8)].
In clinical trials of OLYSIO, modest increases in bilirubin levels were observed without impacting hepatic function [see Adverse Reactions (6.1)]. Postmarketing cases of hepatic decompensation with markedly elevated bilirubin levels have been reported. Monitor liver chemistry tests before and as clinically indicated during OLYSIO combination therapy. Patients who experience an increase in total bilirubin to greater than 2.5 times the upper limit of normal should be closely monitored:
· Patients should be instructed to contact their healthcare provider if they have onset of fatigue, weakness, lack of appetite, nausea and vomiting, jaundice or discolored feces.
· Discontinue OLYSIO if elevation in bilirubin is accompanied by liver transaminase increases or clinical signs and symptoms of hepatic decompensation.
The Indications and Usage section was updated to state:
Limitations of Use:
· OLYSIO is not recommended in patients with moderate or severe hepatic impairment (Child Pugh Class B or C) [see Dosage and Administration (2.5), Warnings and Precautions (5.2), Adverse Reactions (6.1, 6.2), Use in Specific Populations (8.8) and Clinical Pharmacology (12.3)].
The Dosage and Administration section was updated as follows:
2 DOSAGE AND ADMINISTRATION
2.1 OLYSIO Combination Treatment
Administer OLYSIO in combination with other antiviral drugs for the treatment of CHC infection. Monitor liver chemistry tests before and during OLYSIO combination therapy [see Warnings and Precautions (5.2)]. OLYSIO monotherapy is not recommended. For specific dosing recommendations for the antiviral drugs used in combination with OLYSIO, refer to their respective prescribing information.
2.5 Hepatic Impairment
OLYSIO is not recommended for patients with moderate or severe hepatic impairment (Child Pugh Class B or C) [see Use in Specific Populations (8.8)]. There have been postmarketing reports of hepatic decompensation, hepatic failure, and death in patients with advanced or decompensated cirrhosis receiving OLYSIO combination therapy [see Warnings and Precautions (5.2) and Adverse Reactions (6.2)]. Simeprevir exposures are increased in patients with moderate or severe hepatic impairment (Child-Pugh Class B or C) [see Clinical Pharmacology (12.3)].
In clinical trials, higher simeprevir exposures have been associated with increased frequency of adverse reactions, including increased bilirubin, rash and photosensitivity [see Adverse Reactions (6.1)].
OLYSIO in combination with Peg IFN alfa and RBV is contraindicated in patients with decompensated cirrhosis (moderate or severe hepatic impairment) [see Peg IFN alfa prescribing information].
The Adverse Reactions section was updated as follows:
6 ADVERSE REACTIONS
Laboratory abnormalities
Elevations in bilirubin were predominately mild to moderate (Grade 1 or 2) in severity, and included elevation of both direct and indirect bilirubin. Elevations in bilirubin occurred early after treatment initiation, peaking by study Week 2, and were rapidly reversible upon cessation of OLYSIO. Bilirubin elevations were generally not associated with elevations in liver transaminases. The frequency of elevated bilirubin was higher in subjects with higher simeprevir exposures.
6.2 Postmarketing Experience
The following adverse reactions have been reported during post approval use of OLYSIO. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship between drug exposure and these adverse reactions.
Cardiac Disorders: Serious symptomatic bradycardia has been reported in patients taking amiodarone who initiate treatment with sofosbuvir in combination with another HCV direct acting antiviral, including OLYSIO [see Warnings and Precautions (5.1) and Drug Interactions (7.3)].
Hepatobiliary Ddisorders: hepatic decompensation, hepatic failure [see Warnings and Precautions (5.2)].
Section 7 Drug Interactions was updated to include revisions to the antiarrhythmic section.
Section 8 Use in Specific Populations was updated as follows:
8 USE IN SPECIFIC POPULATIONS
8.8 Hepatic Impairment
No dose adjustment of OLYSIO is required in patients with mild hepatic impairment (Child Pugh Class A) [see Clinical Pharmacology (12.3)].
The safety and efficacy of OLYSIO have not been established in HCV infected patients with moderate or severe hepatic impairment (Child Pugh Class B or C).
OLYSIO is not recommended for patients with moderate or severe hepatic impairment (Child Pugh Class B or C) [see Dosage and Administration (2.5)].
There have been postmarketing reports of hepatic decompensation, hepatic failure, and death in patients with advanced or decompensated cirrhosis receiving OLYSIO combination therapy [see Warnings and Precautions (5.2) and Adverse Reactions (6.2)]. Simeprevir exposures are increased in patients with moderate or severe hepatic impairment (Child Pugh Class B or C) [see Clinical Pharmacology (12.3)]. In clinical trials, higher simeprevir exposures have been associated with increased frequency of adverse reactions, including increased bilirubin, rash and photosensitivity [see Adverse Reactions (6.1)].
Refer to the prescribing information for the antiviral drug(s) used in combination with OLYSIO regarding their use in patients with hepatic impairment.
The combination of OLYSIO with Peg IFN alfa and RBV is contraindicated in patients with decompensated cirrhosis (moderate or severe hepatic impairment).
Section 12.3 Pharmacokinetics included the following updates:
12.3 Pharmacokinetics
Specific Populations
Hepatic Impairment
Simeprevir is primarily metabolized by the liver. Compared to HCV uninfected subjects with normal hepatic function, the mean steady state AUC of simeprevir was 2.4 fold higher in HCV uninfected subjects with moderate hepatic impairment (Child Pugh Class B) and 5.2 fold higher in HCV uninfected subjects with severe hepatic impairment (Child Pugh Class C).
No dose adjustment of OLYSIO is necessary in patients with mild hepatic impairment (Child Pugh Class A).
The safety and efficacy of OLYSIO have not been established in HCV infected patients with moderate or severe hepatic impairment (Child Pugh Class B or C).
OLYSIO is not recommended for use in patients with moderate or severe hepatic impairment (Child Pugh Class B or C) [see Dosage and Administration (2.5), Warnings and Precautions (5.2) and Use in Specific Populations (8.8)].
In clinical trials, higher simeprevir exposures have been associated with increased frequency of adverse reactions, including increased bilirubin, rash and photosensitivity [see Adverse Reactions (6.1)].
Based on a population pharmacokinetic analysis of HCV infected subjects with mild hepatic impairment (Child-Pugh Class A) treated with OLYSIO, liver fibrosis stage did not have a clinically relevant effect on the pharmacokinetics of simeprevir.
Refer to the prescribing information for the antiviral drugs used in combination with OLYSIO regarding their use in patients with hepatic impairment.
Drug Interactions
[See also Warnings and Precautions (5.67) and Drug Interactions (7)]
In vitro studies indicated that simeprevir is a substrate and mild inhibitor of CYP3A. Simeprevir does not affect CYP2C9, CYP2C19 or CYP2D6 in vivo. Simeprevir does not induce CYP1A2 or CYP3A4 in vitro. In vivo, simeprevir mildly inhibits the CYP1A2 activity and intestinal CYP3A4 activity, while it does not affect hepatic CYP3A4 activity. Simeprevir is not a clinically relevant inhibitor of cathepsin A enzyme activity.
In vitro, simeprevir is a substrate for P gp, MRP2, BCRP, OATP1B1/3 and OATP2B1; simeprevir inhibits the uptake transporters OATP1B1/3 and NTCP and the efflux transporters P gp/MDR1, MRP2 and BSEP. The inhibitory effects of simeprevir on the bilirubin transporters OATP1B1/3 and MRP2 likely contribute to clinical observations of elevated bilirubin [see Adverse Reactions (6.1)].
The updated product label will be available soon at DailyMed or at Drugs@FDA.
Richard Klein
Office of Health and Constituent Affairs
Food and Drug Administration
Kimberly Struble
Division of Antiviral Drug Products
Food and Drug Administration
Steve Morin
Office of Health and Constituent Affairs
Food and Drug Administration
|
|
| |
| |
|
|
|