| |
Daclatasvir in Combination With Asunaprevir and Beclabuvir for
Hepatitis C Virus Genotype 1 Infection With Compensated Cirrhosis
|
| |
| |
Download the PDF here
Andrew J. Muir, MD1; Fred Poordad, MD2; Jacob Lalezari, MD3; Gregory Everson, MD4; Gregory J. Dore, MBBS, MPH, PhD5,6; Robert Herring, MD7; Aasim Sheikh, MD8; Paul Kwo, MD9; Christophe Hezode, MD, PhD10; Paul J. Pockros, MD11; Albert Tran, MD, PhD12,13; Joseph Yozviak, DO14; Nancy Reau, MD15; Alnoor Ramji, MD16; Katherine Stuart, MBBS, PhD17; Alexander J. Thompson, MBBS, PhD18,19; John Vierling, MD20; Bradley Freilich, MD21; James Cooper, MD22; Wayne Ghesquiere, MD23; Rong Yang, PhD24; Fiona McPhee, PhD25; Eric A. Hughes, MD, PhD24; E. Scott Swenson, MD, PhD25; Philip D. Yin, MD, PhD25
JAMA. May 5, 2015
ABSTRACT
Importance Effective and well-tolerated, interferon-free regimens are needed for treatment of patients with chronic hepatitis C virus (HCV) infection and cirrhosis.
Objective All-oral therapy with daclatasvir (nonstructural protein 5A [NS5A] inhibitor), asunaprevir (NS3 protease inhibitor), and beclabuvir (nonnucleoside NS5B inhibitor), with or without ribavirin, was evaluated in patients with HCV genotype 1 infection and compensated cirrhosis.
Design, Setting, and Participants The UNITY-2 study was conducted between December 2013 and October 2014 at 49 outpatient sites in the United States, Canada, France, and Australia. Patients were treated for 12 weeks, with 24 weeks of follow-up after completion of treatment. Adult patients with cirrhosis were enrolled in 2 cohorts: HCV treatment-naive or HCV treatment-experienced.
Statistical analyses were based on historical controls; there were no internal controls.
Interventions All patients received twice-daily treatment with the fixed-dose combination of daclatasvir (30 mg), asunaprevir (200 mg), and beclabuvir (75 mg). In addition, patients within each cohort were stratified according to HCV genotype 1 subtype (1a or 1b) and randomly assigned (1:1) to receive double-blinded weight-based ribavirin (1000-1200 mg/d) or matching placebo.
Main Outcomes and Measures Sustained virologic response at posttreatment week 12 (SVR12).
Results One hundred twelve patients in the treatment-naive group and 90 patients in the treatment-experienced group were treated and included in the analysis. Enrolled patients were 88% white with a median age of 58 years (treatment-naive group) or 60 years (treatment-experienced group); 74% had genotype 1a infection. SVR12 rates were 98% (97.5% CI, 88.9%-100%) for patients in the treatment-naive group and 93% (97.5% CI, 85.0%-100.0%) for those in the treatment-experienced group when ribavirin was included in the regimen. With the fixed-dose combination alone, response rates were 93% (97.5% CI, 85.4%-100.0%) for patients in the treatment-naive group and 87% (97.5% CI, 75.3%-98.0%) for those in the treatment-experienced group. Three serious adverse events were considered to be treatment related and there were 4 adverse event–related discontinuations. Treatment-emergent grade 3 or 4 alanine aminotransferase elevations were observed in 4 patients, of which 1 had concomitant total bilirubin elevation.
Conclusions and Relevance In this open-label uncontrolled study, patients with chronic HCV genotype 1 infection and cirrhosis who received a 12-week oral fixed-dose regimen of daclatasvir, asunaprevir, and beclabuvir, with or without ribavirin, achieved high rates of SVR12.
INTRODUCTION
Chronic hepatitis C virus (HCV) infection remains a substantial cause of chronic liver disease, affecting approximately 130 to 150 million individuals worldwide.1 An estimated 20% of patients with chronic HCV infection will develop cirrhosis.2 The prevalence of cirrhosis is increasing, primarily from continuing disease progression in an aging population that acquired HCV infection prior to widespread HCV testing and blood screening.3 Patients with cirrhosis have increased risk of severe sequelae, such as hepatic decompensation, hepatocellular carcinoma, and death.2 Conversely, clearance of HCV infection with effective treatment reduces the risk of disease progression and the need for liver transplantation, and can lead to cirrhosis regression and improved survival.4- 6
Treatment regimens containing peginterferon are problematic for patients with cirrhosis because of reduced response rates and more frequent and severe adverse events.7- 10 Furthermore, patients with cirrhosis more frequently have contraindications for peginterferon such as anemia, renal insufficiency, or thrombocytopenia. Consequently, interferon-free regimens comprising combinations of direct-acting antivirals are being developed to increase response rates and provide viable treatment options for patients with all stages of liver disease.
The all-oral combination of daclatasvir (a potent, pangenotypic nonstructural protein 5A [NS5A] inhibitor), asunaprevir (an NS3 protease inhibitor), and beclabuvir (BMS [Bristol-Myers Squibb]-791325; a nonnucleoside NS5B thumb-1 polymerase inhibitor) was explored in a preliminary study. After 12 weeks of therapy, sustained virologic response at posttreatment week 12 (SVR12) was achieved by 92% of patients with HCV genotype 1 infection in the treatment-naive group, including 13 of the 15 enrolled patients with cirrhosis.11 The present study evaluated this regimen, administered as a twice-daily fixed-dose combination tablet with or without ribavirin, in patients who were treatment-naive and treatment-experienced with chronic HCV genotype 1 infection and compensated cirrhosis.
METHODS
Study Design
The study was conducted between December 2013 and October 2014 at 49 outpatient sites in the United States, Canada, France, and Australia. Two patient cohorts were enrolled: treatment-naive patients with no prior exposure to any interferon formulation or direct-acting antivirals, and treatment-experienced patients who had prior treatment with interferon and/or host-targeted antivirals or direct-acting antivirals other than NS3 or NS5A inhibitors or NS5B thumb-1 inhibitors, which were exclusionary (study protocol appears in Supplement 1).
All patients received open-label daclatasvir (30 mg), asunaprevir (200 mg), and beclabuvir (75 mg) in a fixed-dose combination twice daily (DCV-TRIO). In addition, within each study group, patients were randomly assigned (1:1) to receive blinded weight-based ribavirin (1000-1200 mg/d) or matching placebo twice daily. Patients were treated for 12 weeks with 24 weeks of posttreatment follow-up. Treatment adherence was assessed by patients’ diaries and an accounting of pills. The protocol was approved by the institutional review board or independent ethics committee at each site and all patients provided written informed consent.
Patients
Patients were adults aged 18 years and older with HCV RNA (≥104 IU/mL) and compensated cirrhosis, as defined by liver biopsy demonstrating a METAVIR score of F4, a FibroScan score of greater than 14.6 kPa within the previous year, or a FibroTest score of 0.75 or greater coupled with an aspartate aminotransferase (AST)-to-platelet ratio index of greater than 2 during screening.
Key exclusions included a history of hepatocellular carcinoma, hepatic decompensation events (ascites, hepatic encephalopathy, variceal hemorrhage), active or recent alcohol abuse, coinfection with HIV or hepatitis B virus, body mass index greater than 35 (calculated as weight in kilograms divided by height in meters squared), or other significant medical conditions.
Key laboratory exclusions included an alanine aminotransferase (ALT) or AST level of at least 5 x the upper limit of normal (ULN), total bilirubin of at least 34 μmol/L, international normalized ratio of at least 1.7, an albumin level lower than 3.5 g/dL, or a platelet level of less than 50 x 109 cells/L.
Patient race was self-described as white, black/African American, Asian, American Indian/Alaska native, or other to support assessments of the effect of race on SVR12.
Treatment Assignment and Blinding
After screening and confirmation of eligibility, patients within each cohort were stratified according to genotype 1 subtype (1a vs 1b) and randomly assigned to a study treatment group via an interactive voice-response system using a computer-generated random allocation sequence (Figure 1). Patients, investigators, and the sponsor were blinded to ribavirin treatment assignment and HCV RNA results through posttreatment week 12, with the exception of designated individuals who analyzed pharmacokinetics and drug resistance (statistical analysis plan appears in Supplement 2).
Assessments and End Points
Plasma HCV RNA levels were assayed centrally using the HCV COBAS TaqMan Test version 2.0 (Roche Molecular Systems), with a lower limit of quantitation of 25 IU/mL and a lower limit of detection of approximately 10 IU/mL. HCV RNA levels were determined at baseline; on-treatment weeks 1, 2, 4, 6, 8, and 12; and posttreatment weeks 4, 8, and 12.
HCV genotypes and subtypes were assessed using the RealTime HCV Genotype II assay (Abbott) and the VERSANT HCV genotype 2.0 line probe (LiPA) assay. IL28B genotype (rs12979860 single-nucleotide polymorphism, which predicts treatment response with interferon-based regimens) was determined by polymerase chain reaction amplification and sequencing to assess possible relationships between IL28B genotype and SVR12. NS5A, NS3, and NS5B variants were assessed in all patients with virologic failure and HCV RNA of at least 1000 IU/mL. Virologic failure included on-treatment breakthrough (confirmed HCV RNA increase ≥1 log10 IU/mL from nadir, or HCV RNA increase to ≥25 IU/mL if previously below this level), HCV RNA of at least 25 IU/mL at the end of treatment, or posttreatment relapse (confirmed HCV RNA ≥25 IU/mL when HCV RNA was undetectable at the end of treatment). NS5A polymorphisms were assessed in all patients at baseline by population-based sequencing (sensitivity 20% of the viral population); and NS3 and NS5B polymorphisms were assessed at baseline in a proportion of patients matched by genotype 1 subtype and representing patients with or without subsequent SVR12 in a 2:1 ratio.
Adverse events, clinical laboratory assessments, vital signs, and physical examinations were monitored at all study visits. Patients were also monitored for a protocol-defined end point of ALT (≥3 x ULN) and total bilirubin (≥2 x ULN).
The primary outcome measure was SVR12, defined as HCV RNA less than the lower level of quantitation (25 IU/mL; target detected or not detected) at posttreatment week 12. Key secondary end points included HCV RNA response at each on-treatment and posttreatment study visit; SVR12 by genotype 1 subtype, SVR12 by IL28B genotype; on-treatment frequencies of serious adverse events, adverse event–related discontinuations; and hematologic- or liver-related grade 3 or 4 laboratory abnormalities.
Statistical Analyses
The primary objective was to determine whether the SVR12 rate in at least 1 treatment-naive group (with ribavirin or without ribavirin) was significantly higher than a 69% historical threshold (eAppendix in Supplement 3). This threshold is a composite of response rates achieved in this population with approved direct-acting antivirals combined with peginterferon and ribavirin. A 97.5% CI was applied. The type I error rate was .025 (0.05/2; overall α level .05) for each group within a cohort using Bonferroni multiplicity adjustment for each group and comparing with the historical threshold. The primary objective was achieved if the lower bound of the 97.5% CIs of SVR12 for at least 1 group exceeded the historical threshold. CIs were based on the normal approximation to the binomial distribution unless the sample size was small for a subgroup (<30 patients) or proportions were close to 0% or 100% when exact binomial CIs were used. The target sample size of 50 patients per group of the treatment-naive cohort had a greater than 90% probability to demonstrate that the SVR12 rate in at least 1 group would be higher than the historical threshold, assuming 2-sided 97.5% CIs and true SVR12 rates for both groups were greater than 84%. Data were analyzed using SAS version 9. The primary end point assessment (SVR12) was based on all treated patients.
RESULTS
Patient Disposition and Characteristics
Of 300 patients who were screened, 202 were enrolled and initiated treatment and 98 were not enrolled (of whom 92 were not enrolled because of not meeting study entry criteria) (Figure 1).
Baseline characteristics were similar among treatment groups in each cohort. Overall, patients were 86% white, 74% had genotype 1a infection, 85% had baseline viral loads greater than 800 000 IU/mL, and 73% had IL28B non-CC genotypes (Table 1). The median age was relatively high (58-60 years) compared with other studies in cirrhotic populations.12,13 Cirrhosis was diagnosed by liver biopsy in 108 patients (53%), by meeting FibroScan criteria (>14.6 kPa) in 79 patients (39%), and by meeting FibroTest/AST-to-ratio index criteria in 15 patients (7%). Overall, 53 patients (26%) had baseline platelet counts greater than 100 000 cells/mm3, suggestive of more advanced disease associated with portal hypertension.
In the treatment-experienced cohort, 69 of 90 patients (77%) had previously failed to respond to interferon-based regimens, including 35 (39%) with prior null response; and 16 of 90 patients (18%) were previously intolerant of interferon-based therapy. Five patients had been treated unsuccessfully with regimens containing peginterferon/ribavirin in combination with amantadine, alisporivir, mericitabine, GS9450, thymosin, or milk thistle.
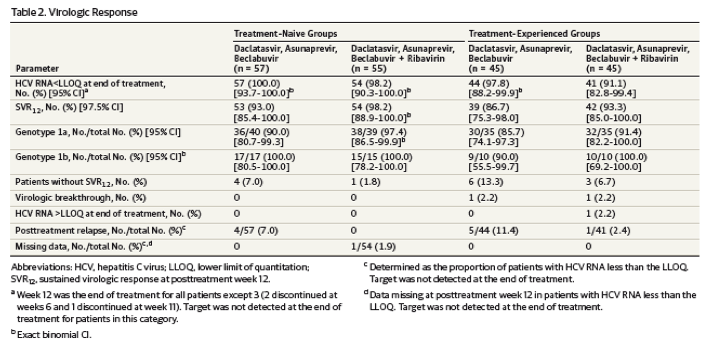
Virologic Response
In the treatment-naive cohort, SVR12 was achieved by 93% (97.5% CI, 85.4%-100.0%) of patients receiving DCV-TRIO alone and by 98.2% (97.5% CI, 88.9%-100.0%) of patients with ribavirin added; and corresponding SVR12 rates for the treatment-experienced cohort were 86.7% (97.5% CI, 75.3%-98.0%) for patients receiving DCV-TRIO alone and 93% (97.5% CI, 85.0%-100.0%) for patients with ribavirin added (Table 2). SVR12 was achieved by 51 of 52 patients (98%) with genotype 1b infection overall; and SVR12 rates in patients with genotype 1a were 86% to 97% across all treatment groups.
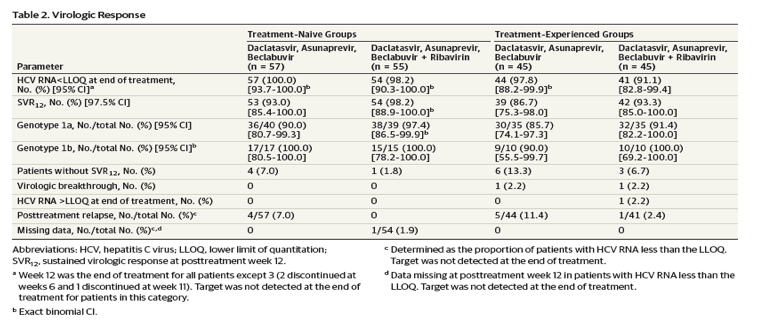
SVR12 was achieved by 50 of 53 patients (94%) with baseline thrombocytopenia (platelets <100 000/mm3), including 23 of 26 patients receiving DCV-TRIO alone and all 27 patients receiving DCV-TRIO with ribavirin. Among patients with baseline platelet levels of less than 75 000/mm3, 10 of 10 patients receiving DCV-TRIO with ribavirin and 8 of 9 patients receiving DCV-TRIO alone achieved SVR12. Among the 35 prior null responders in the experienced cohort, 34 (97%) achieved SVR12, including 18 of 19 patients receiving DCV-TRIO alone and 16 of 16 receiving DCV-TRIO with ribavirin.
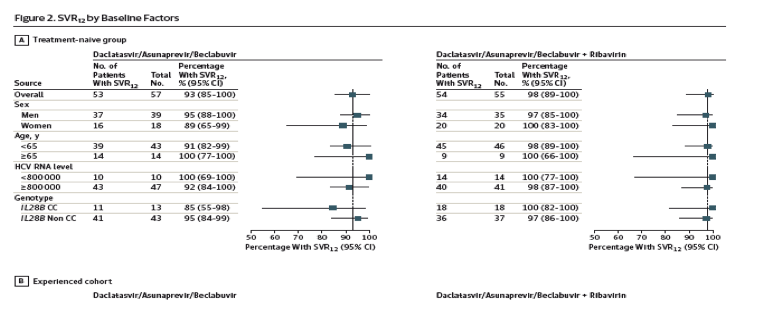
SVR12 rates were comparable according to age, sex, baseline HCV RNA level, and IL28B genotype (Figure 2). Early virologic response was not predictive of treatment failure; among the 62 patients in whom HCV RNA remained detectable at week 4, SVR12 was subsequently achieved in 56 (90%).
Two patients in the treatment-experienced group had on-treatment virologic breakthrough and 1 patient in the treatment-experienced group had HCV RNA detectable at the end of treatment (Table 2). Treatment adherence in these individuals was confirmed by dosing logs. There were no virologic breakthroughs in the treatment-naive cohort. Ten of 202 patients (5%) overall relapsed posttreatment; 9 of these 10 patients received regimens without ribavirin. Two of 13 patients with documented virologic failure had NS5A resistance variants detectable at baseline; 1 patient had a combination of NS5A-Q30K and -Y93H that confers high-level daclatasvir resistance in vitro. Overall, 31 of 33 patients (94%) with baseline NS5A resistance polymorphisms achieved SVR12.
The 12-week course of treatment was completed by 199 of 202 patients (Figure 1). Three patients, all in the treatment-experienced cohort, discontinued participation before week 12 (2 because of virologic breakthroughs at weeks 6 and 11 and 1 at week 6 because of concomitant elevations of ALT and total bilirubin). Based on study medication records, more than 90% of patients were 95% adherent for both dose and duration.
Resistance Analyses
NS5A polymorphisms at amino acid positions 28, 30, 31, or 93 were detected at baseline in 15 of 149 patients (10%) with genotype 1a infection and 13 of 52 patients (25%) with genotype 1b infection. Among these 28 patients, 13 of 15 (87%) with genotype 1a and 13 of 13 with genotype 1b subsequently achieved SVR12. Of the 2 patients in the treatment-experienced group with baseline NS5A variants who relapsed, one had M28V at baseline and the other had Q30H and Y93H at baseline. NS5A-M28V confers no loss in daclatasvir anti-HCV activity in vitro, whereas Q30H-Y93H confers a greater than 1000-fold loss in daclatasvir activity. NS3-Q80L and NS5B-A421V were also detected at baseline in the patient with high-level daclatasvir-resistant variants; however, these polymorphisms confer less than or equal to a 2-fold resistance to asunaprevir or less than or equal to a 3-fold resistance to beclabuvir.14,15 There was no apparent association between detection of NS3-Q80K or NS5B-A421V and virologic outcome; the prevalence at baseline in the overall genotype 1a population of NS3-Q80K (19 of 49 patients tested) and NS5B-421V (10 of 49 patients tested) was similar to the prevalence in genotype 1a patients with virologic failure (6 of 12 patients tested for NS3-Q80K and 4 of 12 patients tested for NS5B-421V).
Emergent resistance variants in patients with virologic failure were similar to those described previously.11 NS5A resistance variants emerged at virologic failure in 11 of 12 patients with genotype 1a; NS5A-Q30 variants were observed frequently (7 of 12 patients). NS3 resistance variants emerged in 10 of 12 patients with genotype 1a; NS3-R155K was detected in all 10 patients. Previously reported NS5B resistance variants emerged in 2 of 12 patients with genotype 1a. NS5B-P495 variants were detected only in 2 patients in the treatment-experienced group with on-treatment failure; these patients also had NS5A and NS3 resistance variants at virologic failure. For the single patient with genotype 1b who relapsed, only NS5A-Y93H was detected at virologic failure, with no signature NS3 or NS5B resistance variants detected.
Adverse Events and Laboratory Abnormalities
Nine patients experienced on-treatment serious adverse events; 3 events were considered treatment related: anemia, aminotransferase and bilirubin elevations, and ribavirin overdose (Table 3). Three patients discontinued ribavirin because of adverse events that included anemia (week 4), hemoglobin reduction (week 3), and cough (week 6). One patient discontinued ribavirin at week 2 because of anemia and discontinued DCV-TRIO at week 6 because of ALT and bilirubin elevations. All 4 patients who discontinued subsequently achieved SVR12. Adverse events commonly associated with ribavirin, primarily fatigue, insomnia, and pruritus, were more frequent in ribavirin-containing groups. Similarly, grade 3 or 4 hemoglobin abnormalities occurred in 5% of patients in ribavirin-containing groups, compared with none in the ribavirin-free groups.
Treatment-emergent ALT elevations of greater than 5 x ULN occurred in 4 patients (2%). In 3 patients, the maximum elevations were less than 10 x ULN; none were associated with bilirubin elevations. All 3 patients completed therapy and the ALT elevations resolved posttreatment. One patient with cirrhosis discontinued ribavirin only at week 2 because of anemia, then discontinued all study medication at week 6 because of a protocol-defined event of concomitant elevations of ALT and total bilirubin. These elevations were maximal at week 6 (ALT, 992 U/L; total bilirubin, 2.4 mg/dL [SI conversions: ALT to μkat/L, multiply by 0.0167; total bilirubin to μmol/L, multiply by 17.104]). ALT normalized 6 weeks after discontinuation and total bilirubin normalized 6 weeks after discontinuation; the patient subsequently achieved SVR12. Six patients had treatment-emergent grade 3 or 4 lipase elevations of which none were associated with abdominal pain or symptomatic pancreatitis.
DISCUSSION
Twelve weeks of treatment with the fixed-dose combination of daclatasvir, asunaprevir, and beclabuvir, with or without ribavirin, achieved an overall SVR12 rate of 93% in patients with genotype 1 infection and compensated cirrhosis. Among patients with genotype 1a infection, SVR12 was achieved by 88% of those receiving the fixed-dose combination alone and by 95% of those with ribavirin added to the regimen. However, the contribution of ribavirin to SVR12 remains uncertain because of the small sample sizes; results suggest that inclusion of ribavirin with the regimen may be considered for patients with genotype 1a infection. Thrombocytopenia may be associated with portal hypertension in patients with cirrhosis; in this study, SVR12 was achieved by 94% of patients with baseline thrombocytopenia, suggesting that SVR remains robust in this difficult-to-treat population with cirrhosis. Sex, age, baseline HCV RNA levels, and IL28B genotype had no significant association with SVR12 rates, consistent with previous results suggesting a reduced effect of baseline factors with high-potency regimens.12,16- 18
An overall 98% SVR12 rate was achieved after 12 weeks of treatment in patients with genotype 1b infection and cirrhosis. Factors contributing to the single virologic failure (relapse) with genotype 1b are uncertain; the only resistance variant detected at virologic failure (NS5A-Y93H) conferred only low-level resistance to daclatasvir. Previously, the combination of daclatasvir and asunaprevir, without beclabuvir or ribavirin, achieved SVR12 rate in 83% of a similar genotype 1b population following 24 weeks of treatment.19 A direct comparison of these regimens could confirm whether the addition of beclabuvir reduces virologic failure and increases SVR12 in patients with genotype 1b infection and cirrhosis.
Emergent resistance variants in patients with virologic failure were similar to those reported previously.11 Variants at NS5A-Q30, NS3-R155K, and NS5B-P495 were observed although NS5B variants were not detected in patients experiencing relapse. Overall, resistance variants at baseline were infrequent in this study and did not appear to have an adverse effect on SVR12 rates, in contrast with previous results for the combination of daclatasvir and asunaprevir (without beclabuvir or ribavirin), in which the presence of NS5A-L31, NS5A -Y93, and/or NS3-D168 polymorphisms at baseline reduced SVR12 in genotype 1b-infected patients.19
The regimen was well-tolerated with or without ribavirin, with low rates of treatment-related serious adverse events, adverse event–related discontinuations, or grade 3 or 4 laboratory abnormalities. A single patient met protocol-defined discontinuation criteria of concomitant ALT and bilirubin elevations; the elevations resolved after discontinuation. ALT elevations have been associated with asunaprevir in other combination regimens but are only rarely associated with changes in other liver function test results.19- 21
In trials of other all-oral regimens in patients with cirrhosis, high SVR rates have been achieved, although maximizing SVR rates in this population often requires extended (24 weeks) therapy and/or the inclusion of ribavirin. SVR12 rates comparable with those of the present study were reported for a population of patients with cirrhosis treated with ritonavir-boosted paritaprevir combined with ombitasvir, dasabuvir, and ribavirin.12 In that study, treatment extended to 24 weeks provided a modest increase in SVR12rates, primarily among patients with prior null response to peginterferon/ribavirin. In contrast, 34 of the 35 prior null responders in our study achieved SVR12 after 12 weeks of treatment, suggesting that more extended therapy would have little benefit. In another study that included a limited population of patients with cirrhosis, the combination of sofosbuvir and ledipasvir achieved SVR12 rates of 97% (without ribavirin) and 100% (with ribavirin) in patients with cirrhosis who were treatment naive after 12 or 24 weeks of therapy.17 However, in a parallel study of patients with cirrhosis who were treatment experienced, SVR12 rates were 100% after 24 weeks of therapy but substantially lower (82%-86%) after 12 weeks, leading to the recommendation that patients with cirrhosis receive 24 weeks of therapy.16
Limitations of this study include the absence of a placebo group to support assessment of treatment-related adverse events. The study was not powered to statistically distinguish the contribution of ribavirin to SVR12 rates and the relatively low proportion of black patients limits the extent to which these results can be extrapolated a black population.
CONCLUSIONS
In this open-label, uncontrolled study, patients with chronic HCV genotype 1 infection and cirrhosis who received a 12-week oral fixed-dose regimen of daclatasvir, asunaprevir, and beclabuvir, with or without ribavirin, achieved high rates of SVR12.
|
|
| |
| |
|
|
|