| |
Liver stiffness measurement versus liver biopsy to predict survival and decompensations of cirrhosis among HIV/hepatitis C virus-coinfected patients
|
| |
| |
Download the PDF here
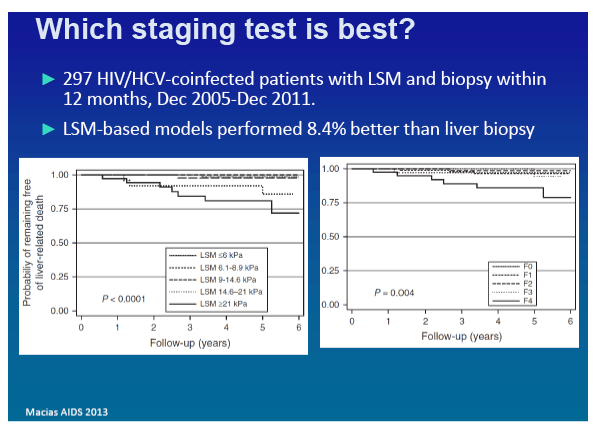
297 HIV/HCV-coinfected patients, who underwent a liver biopsy and LSM
"In this large cohort of patients with near liver biopsy and LSM, we found that LSM-including prognostic models performed similarly to liver biopsy-including models to predict overall mortality in HIV/HCV-coinfected patients. LSM predicted better the likelihood of decompensations of cirrhosis than liver biopsy. The inclusion of liver biopsy in prognostic models improved its yield to predict liver decompensations, but still a trend to better performance was observed for LSM. We believe that the noninvasive nature of LSM might favor the implementation of this technique instead of liver biopsy when predicting the clinical outcome of liver disease in HIV/HCV-coinfection is the only issue.......In summary, the clinical outcome of liver disease in HIV/HCV-coinfected patients can be predicted using LSM instead of liver biopsy. For the prediction of liver decompensations LSM could even improve the performance of liver biopsy. The applicability, safety, and ease of use of liver transient elastography should place this technique as an essential part of the initial evaluation and follow-up of HIV/HCV-coinfected individuals."
Dave Thomas slide, presentation at Ny Course May 8, 2015
----------------------
Liver stiffness measurement versus liver biopsy to predict survival and decompensations of cirrhosis among HIV/hepatitis C virus-coinfected patients
AIDS Oct 23 2013
Macias, Juana; Camacho, Angelab; Von Wichmann, Miguel A.c; Lopez-Cortes, Luis F.d; Ortega, Enriquee; Tural, Cristinaf; Rios, Maria J.g; Merino, Doloresh; Tellez, Franciscoi; Marquez, Manuelj; Mancebo, Mariaa; Pineda, Juan A.a
aInfectious Diseases and Microbiology Unit, Hospital Universitario de Valme, Instituto de Biomedicina de Sevilla, SevillebInfectious Diseases Unit, Instituto Maimonides de Investigacion Biomedica de Cordoba, Hospital Universitario Reina Sofia, CordobacInfectious Diseases Unit, Hospital Donostia, San SebastiandInstituto de Biomedicina de Sevilla, Hospital Universitario Virgen del Rocio/CSIC/Universidad de Sevilla, SevilleeInfectious Diseases Unit, Hospital General Universitario de Valencia, ValenciafInternal Medicine Service, FundacioLluita Contra la SIDA, University Hospital GermansTrias I Pujol, UniversitatAutonoma de Barcelona, BarcelonagInfectious Diseases Unit, Hospital Universitario Virgen Macarena, SevillehInfectious Diseases Unit, Complejo Hospitalario de Huelva, HuelvaiInfectious Diseases Unit, Hospital de La Linea de la Concepcion, CadizjInfectious Diseases Unit, Hospital Universitario Virgen de la Victoria, Malaga, Spain.
Abstract
Objective: To compare the prognostic performance of liver biopsy with that of liver stiffness measurement (LSM) to predict survival and liver decompensations among HIV/hepatitis C virus (HCV)-coinfected patients.
Design: Retrospective cohort study.
Methods: Cohort of 297 HIV/HCV-coinfected patients, who underwent a liver biopsy and LSM separated by 12 months or less, followed in 10 Spanish tertiary care centers from December 2005 to December 2011 (median follow-up, 5 years; interquartile range, 4.2-5.4 years). Liver biopsies were staged following the Scheuer's score. LSM was obtained by hepatic transient elastometry. A survival analysis was carried out and the integrated discrimination improvement was computed to compare the ability of the survival models to predict outcomes. The incidence of death from any cause and of development of the first decompensation of cirrhosis was calculated.
Results: Overall mortality rate was 1.63 [95% confidence interval (CI) 1.06-2.49] per 100 person-years. The adjusted hazard ratio [AHR (95% CI)] of baseline fibrosis (per stage of fibrosis) was 1.52 (1.08-2.15, P = 0.017) and of LSM (per 5 kPa increase) 1.28 (1.12-1.46, P < 0.001). LSM including models yielded a performance 3.9% better than the liver biopsy-based models (P = 0.072). For the prediction of liver decompensations, the AHR (95% CI) of baseline fibrosis by liver biopsy (per stage of fibrosis) was 1.67 (1.15-2.43, P = 0.007) and of LSM (per 5 kPa increase) 1.37 (1.21-1.54, P < 0.001). LSM-based models yielded a performance 8.4% better than the liver biopsy-based models (P = 0.045).
Conclusion: LSM-based prediction achieves a similar yield than liver biopsy-based models to predict overall mortality in HIV/HCV-coinfected patients. Models including LSM could predict better liver decompensations than liver biopsy.
Introduction
Liver disease is a leading cause of death among HIV/hepatitis C virus (HCV)-coinfected patients [1]. The course of chronic hepatitis C is accelerated in HIV infection [2]. Thus, life expectancy is very short once the first decompensation of cirrhosis emerges in HIV/HCV coinfection [3]. Due to these, simple techniques, easy to perform, which can be repeated over time to monitor the progression of fibrosis, are needed for HIV/HCV-coinfected patients. Such tools should allow stratifying the risk of death and liver decompensations in HIV/HCV coinfection.
Survival and decompensations of liver disease in HCV infection are strongly predicted by the fibrosis status [5,6]. Patients with more advanced liver fibrosis are at increased risk for complications from cirrhosis and death [5,6]. Liver biopsy has been considered as the 'gold standard' for defining liver fibrosis [7,8]. However, liver biopsy is limited by morbidity and mortality associated with the procedure, sampling error, intraobserver and interobserver variability, and increased costs [7-9]. In addition, liver biopsy is inadequate to sequentially monitor patients. Liver stiffness measurement (LSM) by transient elastography has improved the ability to define the extent of fibrosis without a liver biopsy [10]. However, not all regulatory agencies have still approved the technique as an alternative liver biopsy. Liver biopsy is without possible replacement by LSM if liver histology assessment is needed to diagnose accompanying lesions. Other limitations include failure to obtain valid LSM in obese patients, and falsely increased values during acute flares of liver enzymes [11]. Finally, LSM is more useful for establishing minimal or absent fibrosis and cirrhosis, but it may be less accurate in assessing the mid-ranges of fibrosis [10,12,13].
In HIV/HCV coinfection, LSM has a high yield to diagnose fibrosis and is accurate to detect cirrhosis [12,13]. Importantly, LSM correlates with the Child-Pugh and the model for end-stage liver disease scores in HIV/HCV-coinfected patients with cirrhosis [14]. Moreover, as a consequence of the correlation between LSM and hepatic venous pressure gradient [15], LSM has been proven to predict absence of significant esophageal varices [16] and decompensations among patients with cirrhosis [17]. These data support a potential role for LSM to replace liver biopsy as prognostic tool. Indeed, multivariate models in patients with chronic hepatitis C without HIV infection including LSM and blood biomarkers showed good yields to predict overall survival [18]. However, there are no data, to our knowledge, comparing directly the prognostic value of liver biopsy and LSM.
The aim of the present study was to compare the prognostic yield of liver biopsy with that of LSM to predict overall survival and decompensations of cirrhosis among HIV/HCV-coinfected patients.
Patients and methods
Patients and follow-up
This was a cohort study that included all HIV-infected patients, seen in 10 Spanish centers from December 2005 to December 2011, who fulfilled the following criteria: HCV infection as determined by a detectable plasma HCV RNA at baseline; a liver biopsy performed as part of the evaluation of HCV infection; and an LSM determination carried out no longer than 12 months around liver biopsy. Patients with metabolic, vascular, biliary, or tumoral liver disease were excluded. All patients fulfilling those criteria were included in the cohort regardless the quality of the biopsy sample or that of the LSM. Within 1 month before the date of LSM evaluation, epidemiological, clinical, and laboratory data were abstracted from database and clinical records. During the follow-up, clinical events as liver decompensations, liver-related, and all-cause deaths were similarly collected. In the participating centers, HIV-infected patients follow scheduled visits, at least, every 3-6 months. The vital status of patients lost to follow-up was established whenever possible through queries to administrative databases, to hospital-based registries, and telephone calls to next of kin proxies.
Liver fibrosis evaluation
Hepatic transient elastography (FibroScan; Echosens, Paris, France) was carried out according to a standardized technique [19] by one experienced operator at each center. LSM was considered as a good quality determination if the interquartile range (IQR) was lower than 30% of the median value of LSM [19]. LSM cutoffs previously validated in HIV/HCV-coinfected individuals were considered: LSM less than 6 kPa, indicative of mild or absent fibrosis [12]; LSM 9 kPa at least, suggestive of liver fibrosis extended beyond the portal tracts [12]; LSM 14.6 kPa at least, diagnostic of cirrhosis [13]; less than 21 kPa, absence of esophageal varices needing prophylaxis against bleeding [16]. These cutoffs were applied to define the following ranges of LSM: less than 6 kPa, 6.1-8.9 kPa, 9-14.5 kPa, 14.6-21, more than 21 kPa.
Liver biopsies were staged following the Scheuer index [20] by one experienced pathologist at each center as: 0, absent fibrosis; 1, portal fibrotic expansion; 2, extension of fibrosis to the lobule, but with few septa; 3, bridging fibrosis with numerous septa, with architectural distortion without cirrhosis; 4, cirrhosis. Biopsies with length equal or longer than 15 mm were considered as adequate.
Clinical management
Patients received antiretroviral therapy (ART) according to the availability of drugs and the recommendations of international guidelines and panels of experts in force during the study period. Therapy against HCV infection was prescribed according to the caring physician criteria, based on consensus recommendations in effect along the study period, usually guided by HCV genotype and liver fibrosis stage. HCV therapy available during the study period was the combination of pegylated interferon and ribavirin. Sustained virological response (SVR) was defined as undetectable serum HCV RNA 24 weeks after the end of treatment.
Individuals with the diagnosis of cirrhosis were included in programs to screen hepatocellular carcinoma (HCC) and esophageal varices. The following clinical events were classified as decompensations of cirrhosis: spontaneous bacterial peritonitis, portal hypertensive gastrointestinal bleeding, ascites, hepatorenal syndrome, hepatic encephalopathy, nonobstructive jaundice, and HCC. The diagnosis and management of individuals with cirrhosis and of liver decompensations is described elsewhere [4].
Statistical analysis
The primary end-points were death from any cause and the development of the first decompensation of cirrhosis. The secondary end-point was liver-related death. The date of inclusion in the cohort, the baseline date, was the day corresponding to half the period between the date of liver biopsy and the date of LSM determination. The time to the event was the length of time since baseline until the end-point. For patients who did not die or develop a liver decompensation, the analysis was censored at the date of their last clinical visit or May 2012. The relationship between the emergence of the study end-points and baseline liver fibrosis staged by biopsy or baseline LSM were evaluated. The associations between the time to death or to the first liver decompensation and the following baseline factors were also examined: age, sex, AIDS, HIV viral load, CD4+ cell counts, use of ART, alanine aminotransferase (ALT) serum levels, platelet counts, and achievement of SVR. The time to event was evaluated using Kaplan-Meier curves. Survival curves were compared applying the log-rank test. In order to assess whether LSM and fibrosis staged by biopsy independently predicted the primary end-points, factors with a level of association P ≤ 0.2 in univariate analyses were included in Cox's regression models. Proportional hazard assumptions were checked by use of Schoenfeld's residuals. For liver decompensations, factors with a univariate P-value ≤ 0.2 were included in competing risks regression models, wherein deaths not because of liver failure were considered competing events. Multivariate models were adjusted for age and sex. Adjusted associations with P ≤ 0.05 were considered as significant. The performance of single variables and multivariate models to predict primary end-points was assessed by receiver-operating characteristic curves (ROCs). Areas under the ROC (AUROC) of models including baseline liver fibrosis in the biopsy and those including baseline LSM were compared applying the Hanley and McNeil test. The integrated discrimination improvement (IDI) was computed to compare the ability of the models to predict outcomes [21]. Aside from the primary analysis, three additional sensitivity analyses were carried out restricting the analysis to patients with adequate liver biopsy or LSM, and to individuals with persistent HCV infection, that is, without SVR. Data were analyzed using SPSS 15.0 (SPSS Inc., Chicago, Illinois, USA) and Stata SE 9.0 (Statacorp, College Station, Texas, USA).
Ethical aspects
This study has been designed and performed according to the Helsinki declaration and was approved by the Ethics Committee of Hospital Universitario de Valme.
Results
Characteristics of the cohort
During the study period, 297 HIV-infected patients met the inclusion criteria for this cohort. Most patients were former IDUs. None of the patients was an active IDU at the time of fibrosis evaluation. At baseline, the majority of patients were on effective ART, showing undetectable plasma HIV RNA and high CD4+ cell counts (Table 1). Individual antiretroviral drugs at baseline are showed in Table 1. The most prevalent HCV genotypes were 1 and 4. Over two-thirds of the patients received pegylated interferon and ribavirin during their follow-up. Other characteristics of the cohort are summarized in Table 1. The median (IQR) follow-up of the cohort was 5 (4.2-5.4) years. Twenty-six (8.8%) patients were lost to follow-up.
Overall mortality and liver-related deaths
Twenty-one [7.1%, 95% confidence interval (CI) 4.1-10%] patients died during the follow-up. None of the deaths was attributable to AIDS, 12 (57%) deaths were liver-related and 9 (43%) were due to other causes. The causes of death other than liver-related were suicide, sudden death, sepsis, each in one patient, opiate overdose in two individuals, and non-AIDS-related cancer in three patients. The overall mortality rate was 1.56 (95% CI 1.02-2.40) deaths per 100 person-years. The probability of survival at 1 year was 97% (94-98%), 93% (89-96%) at 3 years and 88% (81-93%) at 5 years. Mortality rates according to the baseline stage of fibrosis by biopsy and LSM cutoff values are showed in Table 2. The probability of death was significantly higher for individuals with more advanced fibrosis stage by biopsy (Fig. 1a). Similarly, increasing LSM categories were associated with shorter survival (Fig. 1b). The AUROC (95% CI) of liver fibrosis staged by biopsy to predict overall deaths was 0.655 (0.516-0.794) and that of LSM was 0.771(0.655-0.888) (P = 0.055).
The liver-related death rate was 0.93 (95% CI 0.53-1.64) deaths per 100 person-years. The probability of survival free of liver-related death at 1 year was 99% (97-100%), 97% (94-98%) at 3 years and 96% (92-98%) at 5 years.
Liver-related death rates by baseline stage of fibrosis by biopsy and by LSM cutoff values are showed in Table 2. The probability of liver-related death was higher for those with cirrhosis staged by biopsy (Fig. 1c). Higher LSM categories were associated with a higher likelihood of liver-related death (Fig. 1d). The AUROC (95% CI) of liver fibrosis staged by biopsy to predict overall deaths was 0.672 (0.481-0.863) and that of LSM was 0.843 (0.711-0.976) (P = 0.040).
Univariate survival analysis is summarized in Table 3. Two Cox's regression models were elaborated. One of them including baseline liver fibrosis staged by biopsy and the other one including baseline LSM (Table 3). The AUROC (95% CI) of the model based on liver fibrosis staged by biopsy was 0.784 (0.688-0.879) and that of the model including LSM was 0.803 (0.700-0.906) (P = 0.539). Finally, assessment of the IDI indicated that the LSM including models yielded a performance 3.9% better than the models based on liver fibrosis staged by biopsy (P = 0.072).
For the first secondary sensitivity analysis, patients in whom liver biopsy length was equal or longer than 15 mm in 210 (71%) individuals were selected. For this group of patients, the overall adjusted hazard ratio [AHR (95% CI)] for death of liver fibrosis evaluated by biopsy was 1.48 (0.99-2.22). For the second secondary sensitivity analysis, 251 (85%) individuals with LSM IQR was lower than 30% of the median LSM value were selected. Among these individuals, the AHR (95% CI) for overall death of LSM was 1.33 (1.13-1.56). With this sensitivity analysis, the AUROC (95% CI) of the model based on liver fibrosis by biopsy was 0.772 (0.667-0.878) and the AUROC (95% CI) of the model including LSM was 0.800 (0.698-0.903) (P = 0.316). Finally, the third secondary sensitivity analysis was that restricted to patients without SVR. Among them, the AUROC (95% CI) of the model based on liver fibrosis by biopsy was 0.740 (0.621-0.860) and the AUROC (95% CI) of the model including LSM was 0.783 (0.666-0.900) (P = 0.243).
Decompensations of cirrhosis
Twenty-one (7.1%, 95% CI 4.1-10%) patients suffered a liver decompensation during the follow-up. Twelve (57%) of them developed ascites as the first manifestation of end-stage liver disease. Portal hypertensive gastrointestinal bleeding as the first decompensation of liver disease emerged in four (19%) patients, hepatoencephalopathy in two (9.5%), jaundice in two (9.5%), and spontaneous bacterial peritonitis in one (4.8%). The liver decompensation rate was 1.59 (95% CI 1.03-2.43) decompensations per 100 person-years. The probability of remaining free of liver decompensation at 1 year was 96% (92-98%), 93% (89-95%) at 3 years and 89% (82-93%) at 5 years. Liver decompensation rates according to the baseline stage of fibrosis by biopsy and by LSM cutoff values are showed in Table 4. The cumulative incidence of the first liver decompensation by biopsy fibrosis stage and LSM cutoff values are showed in Fig. 2. The AUROC (95% CI) of biopsy-staged liver fibrosis to predict the first decompensation of cirrhosis was 0.713 (0.581-0.845) and that of LSM was 0.870 (0.768-0.973) (P = 0.008).
Univariate survival analysis is summarized in Table 4. Two competing risks regression models were elaborated, one including baseline biopsy-staged liver fibrosis and the other one including baseline LSM (Table 4). In the latter model, the only variable independently associated with the emergence of liver events was baseline LSM. The AUROC (95% CI) of the model including biopsy-staged liver fibrosis was 0.774 (0.663-0.885) and that of LSM was 0.869 (0.766-0.972) (P = 0.056). Evaluation of the IDI showed that the LSM-based models yielded a performance 8.4% better than the liver fibrosis staged by biopsy models (P = 0.045).
For the first secondary sensitivity analysis, among patients with liver biopsy length equal or longer than 15 mm, the adjusted subhazard ratio [SHR (95% CI)] for decompensations of cirrhosis of biopsy-staged fibrosis was 1.62 (1.09-2.39).
For the second secondary sensitivity analysis, that restricted to individuals with LSM IQR lower than 30% of the median LSM value, the AHR (95% CI) for decompensations of cirrhosis of LSM was 1.49 (1.27-1.75). In this sensitivity analysis, the AUROC (95% CI) of the model based on liver fibrosis by biopsy was 0.821 (0.724-0.919) and the AUROC (95% CI) of the model including LSM was 0.901 (0.806-0.996) (P = 0.192). For the third secondary sensitivity analysis, restricted to patients with persistent HCV infection, the AUROC (95% CI) of the model based on liver fibrosis by biopsy was 0.808 (0.710-0.907) and the AUROC (95% CI) of the model including LSM was 0.905 (0.830-0.980) (P = 0.053).
Discussion
In this large cohort of patients with near liver biopsy and LSM, we found that LSM-including prognostic models performed similarly to liver biopsy-including models to predict overall mortality in HIV/HCV-coinfected patients. LSM predicted better the likelihood of decompensations of cirrhosis than liver biopsy. The inclusion of liver biopsy in prognostic models improved its yield to predict liver decompensations, but still a trend to better performance was observed for LSM. We believe that the noninvasive nature of LSM might favor the implementation of this technique instead of liver biopsy when predicting the clinical outcome of liver disease in HIV/HCV-coinfection is the only issue.
Reasons to perform a liver biopsy comprise primarily to provide information on the current status of liver injury, to identify features useful in the decision to embark on therapy, and to detect advanced fibrosis or cirrhosis that necessitates specific screening for HCC and esophageal varices [7,8]. Thus, guidelines state the recommendation of considering liver biopsy in patients with chronic hepatitis C to gather information for prognostic purposes and for treatment decision [7,8]. Guidelines position on the role of liver biopsy is to consider the procedure as the current standard to assess disease severity. Noninvasive tests are regarded either as not able to replace liver biopsy [8] or as alternatives to liver biopsy that may reduce the necessity of it [7]. Given that liver fibrosis staging is aimed at foreseeing clinical events that may ultimately shorten survival and require therapy, we provide data that support the replacement of liver biopsy with hepatic transient elastography as prognostic tool. However, whenever an additional histological liver damage is suspected liver biopsy will be needed to clarify the cause. The performance of LSM to predict hard clinical end-point was similar or better than that of fibrosis staged by liver biopsy in the present study. As a consequence, clinical management decisions can be safely made using LSM instead of liver biopsy. The lack of invasiveness, simplicity, and ease for repeated determinations over time favor the use of LSM.
The prognostic yield for liver decompensations of liver biopsy compared with LSM was significantly lower. After adjustment for baseline characteristics and response to therapy against HCV infection, the AUROCs of fibrosis staged by liver biopsy and LSM were closer, but still there was a trend for better performance of LSM. A sensitivity analysis including only patients without SVR, and therefore with persistent HCV infection throughout the follow-up, showed similar results. A nonsignificant difference between AUROCs for the prediction of decompensations of cirrhosis was only observed after excluding patients with liver biopsy samples and LSM of lower quality. However, daily clinical decisions are often made on the basis of the available data. Liver biopsy information is usually not disregarded by clinicians because of less quality samples.
Low-quality biopsy samples are frequent. In the present study, one-third of the patients had liver biopsy lengths smaller than 15 mm. This figure was even higher in some cohorts [6,22] and clinical trials [5]. Importantly, shorter biopsy samples can lead to understaging of liver fibrosis [5,9]. This was probably the reason behind the improved performance of longer liver biopsies to predict decompensations of cirrhosis. The frequency of inadequate LSM was lower, and the exclusion of patients with inadequate LSM had less influence on the prognostic value of the technique. Thus, in real-life conditions of use, LSM is more reliable than liver biopsy to predict liver events.
This study may have a few limitations. First, recruitment of patients was limited within a time frame when both liver biopsy and LSM were performed in our clinical practice. This precludes the inclusion of patients with more prolonged follow-up, in whom the probability of clinical events could be higher. However, the study follow-up was long, approaching the length of the hepatitis C antiviral long-term treatment against cirrhosis trial [4]. This allowed us to detect enough incident events and deaths to compare the prognostic performance of liver biopsy versus LSM. Second, liver biopsy is not universally offered to HIV/HCV-infected patients. In the present study, patients underwent a liver biopsy as candidates of therapy against HCV. Thus, patients with a worse clinical profile might have been excluded from the study. This potential issue does not affect the study results. The performance of two techniques was compared among patients who were candidates to them in real life. Third, retrospective assessment of patients might be a source of data loss. However, clinical end-points as death and decompensations of cirrhosis are not easily missed in databases or clinical records, thus it is unlikely that the retrospective design might have influenced the results.
Direct-acting antiviral (DAA) agents to treat HCV infection substantially increase the rates of SVR both in naive [23,24] and in treatment-experienced patients [25,26]. The first protease inhibitors, telaprevir and boceprevir, have been approved and many more DAA drugs are underway. It has been proposed that clinicians may consider expanding the pool of patients treated [27]. High rates of SVR among treatment-naive patients and a shorter duration of therapy are possible for most. Thus, treating a broader population of patients, that is, those with milder disease, could improve the risk/benefit ratio of therapy [27]. However, DAA drugs are limited by increased costs. For publicly funded health insurance systems, it could be hard to offer the currently available DAA agent-based therapy for all potential candidates. A rational approach for the selection of patients is needed. LSM assessment can aid decisions on the grounds of the prognostic evidence herein reported. Previously validated LSM cutoffs can be applied for prioritization of therapy with DAA drugs. Increases over time of LSM could also be used, as each 5 kPa raise was associated with a 1.5-fold increase in the risk of liver decompensation in the present study.
In summary, the clinical outcome of liver disease in HIV/HCV-coinfected patients can be predicted using LSM instead of liver biopsy. For the prediction of liver decompensations LSM could even improve the performance of liver biopsy. The applicability, safety, and ease of use of liver transient elastography should place this technique as an essential part of the initial evaluation and follow-up of HIV/HCV-coinfected individuals.
|
|
| |
| |
|
|
|