| |
Noninvasive Methods to Assess Liver Disease in Patients With Hepatitis B or C
|
| |
| |
Download the PDF here
Gastroenterology 2012
Laurent Castera
Department of Hepatology, Hopital Beaujon, Assistance Publique-Hopitaux de Paris, Inserm U773 CRB3, Universite Denis Diderot Paris-7, Clichy, France
---------------------------
Transient elastography: a new surrogate marker
of liver fibrosis influenced by major changes of transaminases.......
http://www.natap.org/2014/HCV/063014_03.htm
Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: A multicenter prospective study (the FIBROSTIC study)......http://www.natap.org/2010/HCV/120510_01.htm
Fibroscan (ultra-sound hepatic elastography)
Review.......http://www.natap.org/2013/HCV/060413_02.htm
ARFI, FibroScan, ELF, and their combinations in the assessment of liver fibrosis: A prospective
study......http://www.natap.org/2012/HCV/071712_03.htm
Diagnosis of Hepatic Fibrosis and Cirrhosis by FibroScan, Transient Elastography, in HIV/Hepatitis C Virus-Coinfected
Patients.......http://www.natap.org/2006/HCV/012006_02.htm
Fibroscan Performance/FDA Approved......http://www.natap.org/2013/HCV/060413_03.htm........http://usa.healthcare.siemens.com/ultrasound/arfi-elastography?stc=wwhcx800013&s_kwcid=AL!91!3!65893863911!b!!g!!fibroscan&ef_id=wTJNGKCSAgAAwi4:20150518153759:s
Ultrasound-based Hepatic Elastography Origins, Limitations, and Applications ....... http://www.natap.org/2013/HCV/060413_02.htm
Journal of Clinical Gastroenterology October 2010
CLINICAL REVIEW
Eric B. Cohen, MD* and Nezam H. Afdhal, MD
--------------------------
Dave Thomas slide NY Course May 8 2015
-----------------------------
Noninvasive Methods to Assess Liver Disease in Patients With Hepatitis B or C
Gastroenterology 2012
Laurent Castera
Department of Hepatology, Hopital Beaujon, Assistance Publique-Hopitaux de Paris, Inserm U773 CRB3, Universite Denis Diderot Paris-7, Clichy, France
The prognosis and management of patients with chronic viral hepatitis B and C depend on the amount and progression of liver fibrosis and the risk for cirrhosis. Liver biopsy, traditionally considered to be the reference standard for staging of fibrosis, has been challenged over the past decade by the development of noninvasive methodologies. These methods rely on distinct but complementary approaches: a biologic approach, which quantifies serum levels of biomarkers of fibrosis, and a physical approach, which measures liver stiffness by ultrasound or magnetic resonance elastography. Noninvasive methods were initially studied and validated in patients with chronic hepatitis C but are now used increasingly for patients with hepatitis B, reducing the need for liver biopsy analysis. We review the advantages and limitations of the noninvasive methods used to manage patients with chronic viral hepatitis B or C infection.
It is important to assess liver disease in patients with viral hepatitis B or C, not only to determine prognosis but to identify patients who require antiviral therapy.1, 2, 3, 4, 5 Liver biopsy has traditionally been the standard for evaluation of tissue damage, including fibrosis.6 Histologic staging of liver fibrosis is a combinatorial assessment of amount of fibrosis and architectural disorganization using the Ishak7 and METAVIR8semiquantitative scoring systems. The clinically relevant end points are detection of significant fibrosis (METAVIR, F ≥2 or Ishak, ≥3), which indicates that patients with hepatitis B or C should receive antiviral treatment, and detection of cirrhosis (METAVIR, F4 or Ishak, 5-6), which indicates that patients should be monitored for complications related to portal hypertension and hepatocellular carcinoma (HCC).1, 5
Liver biopsy analysis has several limitations. It is an invasive procedure that is prone to sampling errors and to intra- and interobserver variation.9, 10Recent American Association for the Study of Liver Diseases guidelines recommended a biopsy of at least 2-3 cm in length, obtained with a 16-gauge needle, that contains more than 11 complete portal tracts for adequate staging and grading of diffuse parenchymal disease.11 However, in clinical practice, few percutaneous needle biopsies meet these criteria.
These limitations, as well as powerful virologic tools for determining genotypes and viral load and new antiviral drugs, have rapidly reduced the use of liver biopsy in management of patients with viral hepatitis. The development of noninvasive methods to assess liver fibrosis over the past decade has advanced the practice of hepatology.12 Apart from assessing liver fibrosis, these noninvasive methods could be used in deciding whether to treat a patient or defer antiviral treatment, in monitoring patients' response to treatment and progression of disease, and in determining prognosis. We review methods for noninvasive evaluation of liver fibrosis and discuss their advantages and limitations in managing patients with viral hepatitis B or C.
Methodologies
The performance of a noninvasive diagnostic method is evaluated by calculation of the area under the receiver operator characteristic curve (AUROC), taking liver biopsy as the reference standard. However, biopsy analysis is an imperfect reference standard: taking into account a range of accuracies of the biopsy, even in the best possible scenario, an AUROC >0.90 cannot be achieved even for a perfect marker of liver disease.13
The AUROC can vary based on the prevalence of each stage of fibrosis, described as spectrum bias.14 Spectrum bias has important implications for the study of noninvasive methods, particularly in comparisons of methods across different study populations. If extreme stages of fibrosis (F0 and F4) are over-represented in a population, the sensitivity and specificity of a diagnostic will be higher than in a population of patients that has middle stages of fibrosis (F1 and F2). Several ways of preventing the "spectrum bias" have been proposed including the adjustment of AUROC using the DANA method (standardization according to the prevalence of fibrosis stages; Difference in Advanced [F2-F3-F4] and Nonadvanced [F0-F1] fibrosis) and nonadvanced (F0-F1) fibrosis)15, 16 or the Obuchowski measure (designed for ordinal gold standards).17 What really matters in clinical practice is the number of patients correctly classified by noninvasive methods for a defined end point according to the reference standard (ie, true positive and true negative).
Noninvasive Methods
Fibrosis can be measured noninvasively, based on a "biological" approach (quantifying biomarkers in serum samples) or based on a "physical" approach (measuring liver stiffness). Although these approaches are complementary, they are based on a different rationale. Liver stiffness corresponds to a genuine and intrinsic physical property of liver parenchyma, whereas serum biomarkers indicate several, not strictly liver-specific features of blood that have been associated with fibrosis stage, as assessed by liver biopsy.
Markers of Liver Fibrosis in Serum
Many serum biomarkers have been evaluated for their ability to determine stage of liver fibrosis, mainly in patients with chronic hepatitis C (for review, see Pinzani et al,18 Manning and Afdhal,19 and Castera20). Among the proposed markers, the so-called direct markers reflect the deposition or removal of extracellular matrix in the liver. These include glycoproteins such as serum hyaluronate, laminin, and YKL-40 and collagens such as procollagen III N-peptide and type IV collagen, collagenases, and their inhibitors such as matrix metalloproteases and tissue inhibitory metalloprotease-1. So-called indirect markers include factors that can be measured in routine blood tests, such as the prothrombin index, platelet count, and ratio of aspartate aminotransferase (AST) to alanine aminotransferase (ALT), which indicate alterations in hepatic function. Results from measurements of direct and indirect markers can be combined and used in diagnosis; the FibroTest (proprietary formula; Biopredictive, Paris, France) was the first algorithm that combined these data.21 Several other scores22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37 have been proposed: 4 are protected by patents and are commercially available (Table 1). Nonproprietary methods use published models, based on routinely available laboratory tests.
The practical advantages of analyzing serum biomarkers to measure fibrosis include their high applicability (>95%) and interlaboratory reproducibility38, 39 and their potential widespread availability (Table 2). However, none are liver specific-their results can be influenced by comorbid conditions, and they require critical interpretation of results. For instance, FibroTest and Hepascore produce false-positive results in patients with Gilbert's syndrome or hemolysis because these patients have hyperbilirubinemia.40 Similarly, acute hepatitis can produce false-positive results in the aspartate-to-platelet ratio index (APRI), Forns index, FIB-4, or Fibrometer tests, which all measure levels of aminotransferases.
Measuring Liver Stiffness
Transient elastography
Liver fibrosis can be staged using 1-dimensional ultrasound transient elastography (TE),41 which measures the velocity of a low-frequency (50 Hz) elastic shear wave propagating through the liver. This velocity is directly related to tissue stiffness, called the elastic modulus (expressed as E = 3pv2, where v is the shear velocity, and p is the density of tissue, assumed to be constant). The stiffer the tissue, the faster the shear wave propagates. TE measures liver stiffness in a volume that approximates a cylinder that is 1-cm wide and 4-cm long, 25-65 mm below skin surface. The results are expressed in kilopascals (kPa) and range from 2.5 to 75 kPa; a normal value is around 5 kPa.42, 43, 44
Advantages to TE include a short procedure time (<5 minutes), immediate results, and the ability to perform the test at the bedside or in an outpatient clinic: it is not a difficult procedure to learn (Table 2). However, accurate results45, 46 require careful interpretation of data, based on at least 10 validated measurements, a success rate (the ratio of valid measurements to the total number of measurement) above 60%, and an interquartile range (IQR; reflects variations among measurements) of less than 30% of the median value (IQR/M, ≤30%).47 Although TE analysis has excellent inter- and intraobserver agreement,48, 49 its applicability (80%) is not as good as that of serum biomarkers. Failure to obtain any measurement has been reported in 3% of cases, and unreliable results (not meeting manufacturer's recommendations) have been reported for 16%,50 mostly because of patient obesity or limited operator experience. A new probe (XL probe; Echosens, Paris, France) has been proposed to overcome these limitations for overweight and obese patients.51 Apart from obese patients, TE results can also be difficult to obtain from patients with narrow intercostal space and are impossible to obtain from patients with ascites.41 The liver is an organ wrapped in a distensible but nonelastic envelope (Glisson's capsula), so additional space-occupying tissue abnormalities, such as edema, inflammation, extrahepatic cholestasis, or congestion, can interfere with measurements of liver stiffness, independently of fibrosis.52 The influence of steatosis is a matter of debate.53, 54
Other imaging methods
Several other liver elasticity-based imaging techniques are being developed, including (2-D) acoustic radiation force impulse imaging (ARFI) and 3-D magnetic resonance (MR) elastography. ARFI involves mechanical excitation of tissue using short-duration (~262 μsec) acoustic pulses that propagate shear waves and generate localized, μ-scale displacements in tissue.55 The shear-wave velocity (expressed in meters/sec) is measured in a smaller region than in TE (10-mm long and 6-mm wide) but can be chosen by the operator. The major advantage of ARFI is that it can be easily implemented on a modified commercial ultrasound machine (Acuson 2000 Virtual Touch Tissue Quantification; Siemens Healthcare, Erlangen, Germany). However, ARFI values, in contrast to TE values, have a narrow range (0.5-4.4 meters/sec). This limits definitions of cut-off values for patient management decisions.
MR elastography uses a modified phase-contrast method to image the propagation characteristics of the shear wave in the liver.56 Elasticity is quantified by MR elastography (expressed in kilopascals) using a formula that determines the shear modulus, which is equivalent to one-third the Young's modulus used with TE.57 The theoretical advantages of MR elastography include its ability to analyze almost the entire liver and its applicability to patients with obesity or ascites. However, MR elastography cannot be performed in livers of patients with iron overload because of signal-to-noise limitations and it is too costly and time-consuming to use in routine practice.
Diagnostic Performance
Quantifying Markers of Liver Fibrosis in Serum
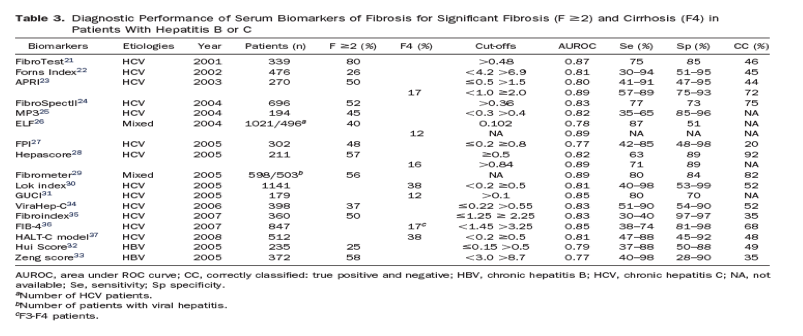
The diagnostic performances of serum biomarkers of fibrosis for significant fibrosis and cirrhosis are summarized in Table 3. Overall, biomarkers are less accurate in detecting intermediate stages of fibrosis than cirrhosis. The most widely used and validated are the APRI (a free nonpatented index) and the FibroTest (a patented test that is not widely available). A meta-analysis by the developer58 that analyzed data from 6378 subjects (individual data from 3282 subjects) who received the FibroTest and biopsies (3501 with hepatitis C virus [HCV] infection and 1457 with hepatitis B virus [HBV]) found that the mean standardized AUROC for diagnosis of significant fibrosis was 0.84, without significant differences between patients with HCV (0.85) and HBV (0.80). Another meta-analysis59 analyzed results from 6259 HCV patients from 33 studies; the mean AUROC values from the APRI in diagnosis of significant fibrosis and cirrhosis were 0.77 and 0.83, respectively. When compared and validated externally in patients with hepatitis C,60, 61, 62, 63, 64, 65 the FibroTest and biopsy analysis had similar levels of performance in diagnosis of significant fibrosis. The largest study (of 913 HCV and 284 HBV patients) prospectively compared the most popular patented tests (FibroTest, Fibrometre, and Hepacore) with the nonpatented test (APRI); the AUROC values for significant fibrosis ranged from 0.72 to 0.78 with no significant differences among scores.64 In patients with cirrhosis, the AUROC values were higher, ranging from 0.77 to 0.86, with no significance in differences. Although nonpatented tests such as the Forns index, FIB-4, and APRI could perform less well, they do not incur additional costs, are easy to calculate, and are available almost everywhere.
Measuring Liver Stiffness
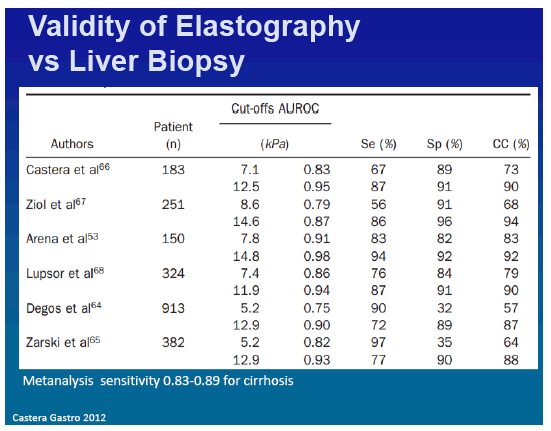
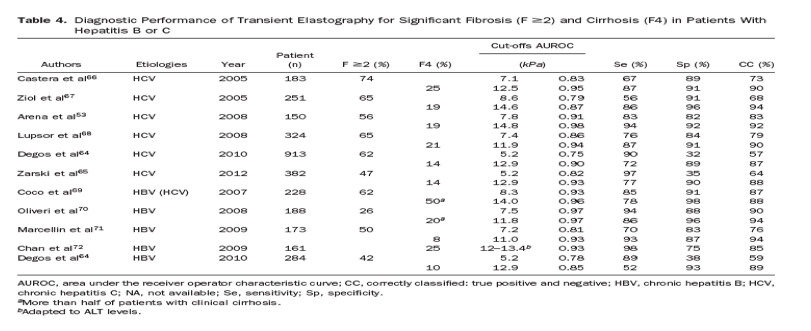
Studies have assessed the ability of TE to quantify liver fibrosis in patients with chronic hepatitis C66, 67 and have been largely confirmed53, 64, 65, 68 and also confirmed in patients with hepatitis B.64, 69, 70, 71, 72 TE more accurately detects cirrhosis (AUROC values, 0.87-0.98; correct classification ranging from 85% to 94%) than significant fibrosis (AUROC values, 0.75-0.93; correct classification from 57% to 90%) (Table 4). Interestingly, proposed cut-off values for cirrhosis ranged from 11 kPa in patients with hepatitis B to 14.8 kPa in patients with hepatitis C. Some researchers have proposed that cut-off values be adapted based on causes of liver disease.73 However, differences among cut-off values could result from differences in prevalence of cirrhosis among study populations (ranging from 8% to 25%). A cut-off value for one population might not be applicable to another, which has a different prevalence of disease. Most studies used single cut-off values for patients with cirrhosis or advanced fibrosis, but more information can be obtained when values are interpreted as a continuum. For example, when liver stiffness values range from 2.5 to 7 kPa, fibrosis is likely mild or absent, whereas when values are above 13 kPa, cirrhosis is likely.47
Several meta-analyses74, 75, 76, 77 have confirmed the better diagnostic performance of TE for cirrhosis than for fibrosis, with mean AUROC values of 0.94 and 0.84, respectively.76 In a meta-analysis of 40 studies (32 papers and 8 abstracts), sensitivity and specificity values were 0.83 and 0.89, respectively, for patients with cirrhosis and 0.79 and 0.78, respectively, for patients with significant fibrosis. However, only 9 studies (comprising 1364 patients) had acceptable standards for liver biopsy and TE, which limit the conclusions. It will therefore be important to perform meta-analyses of data from individual patients.
There are only limited data on the accuracy of ARFI and MR elastography. Preliminary results78 indicate that the accuracy of ARFI is similar to that of TE. However, most studies are based on small samples of heterogeneous populations and do not always use liver biopsy as reference. A single, pilot study of MR elastography (96 patients) reported that it might be more accurate than TE in diagnosis of significant fibrosis,79 but validation is required.
Use in Clinical Practice
Assessing the Stage of Liver Disease
In clinical practice, the determination of fibrosis stage does not need to be as exact as the pathologic scoring system; the absolute stage is less important than determining whether patients have mild or advanced liver disease.
For identifying patients with significant fibrosis, sensitivities and specificities above 85% can be considered sufficient because there are no relevant clinical consequences of false positives or false negatives.80 Because performances of TE and serum biomarkers have been shown to be equivalent,64, 65, 66 the use of either method could depend on local availability. Strategies that combine 2 serum biomarkers,81, 82 or TE and serum biomarkers,66, 83, 84 have been proposed to increase diagnostic accuracy in patients with hepatitis C (Supplementary Figure 1). The advantage of combining 2 unrelated methods, such as TE and biomarkers, over the combination of 2 biomarkers is that TE provides more direct measurement of the liver structure than biomarkers and that there is no relationship between the applicability of TE (success rate and interquartile range) and that of a biomarker.83, 85 Also, the combination of TE and serum biomarkers might be more effective than the combination of 2 biomarkers for detecting significant fibrosis (significantly greater number of saved liver biopsies).86, 87 However, this strategy has only been validated in studies of patients with hepatitis C, is more costly, and could be hampered by the lower applicability of TE, compared with biomarkers.
Identification of patients with cirrhosis requires tests with higher levels of sensitivity because patients might need specific therapies and because patients must be screened for complications. TE appears to be best suited for cirrhosis screening because it has a higher level of performance than biomarker assays64, 84, 88; combining TE with biomarkers does not increase diagnostic accuracy.65, 84, 88 However, the applicability of TE is lower (80% vs 95% for biomarker assays), and the performance levels of these diagnostics might not differ for intention-to-diagnose analysis.65
In summary, the accuracy and applicability of assays for serum biomarkers and TE differ for patients with hepatitis B or C. For example, strategies to combine noninvasive methods have been shown to increase diagnostic accuracy in patients with HCV but have not yet been validated in patients with HBV.89, 90, 91, 92 Serum levels of aminotransferases should also be taken into account in interpreting results from TE in patients with hepatitis B.54 To avoid the risk of false-positive results, some authors have proposed to adapt TE cut-offs based on levels of ALT,72 a strategy that might not apply to patients with fluctuating levels of ALT or hepatitis flares (Table 4). Conversely, in hepatitis e antigen (HBeAg)-negative patients with normal levels of ALT, noninvasive methods, particularly TE, could be used as adjunct tools to measurements of HBV DNA to follow inactive carriers or better identify patients who require liver biopsy (those with ongoing disease activity or significant fibrosis, despite normal levels of ALT).70, 93, 94, 95
Deciding to Provide or Defer Antiviral Therapy
It is important to emphasize that the effects and indications for antiviral treatment differ between patients with hepatitis B and C. For instance, in contrast to hepatitis C, treatment of hepatitis B is not curative and usually prolonged. Apart from fibrosis staging, levels of HBeAg, ALT, and HBV DNA have important roles in treatment decisions for patients with hepatitis B.
In treatment-naïve patients with hepatitis C without comorbidities such as alcoholism or non-alcoholic fatty liver disease, noninvasive methods can be used as first-line assays of fibrosis stage (Figure 1). In that respect, the use of either TE or patented biomarkers (FibroTest, Fibrometer, and Hepascore) was recommended, after an independent systematic review, by the French Health Authorities,96 and recently endorsed by the European Association for Study of Liver Clinical Practice guidelines.5 However, the HCV genotype should also be considered, along with local availability of noninvasive methods and clinical relevance.97 For instance, a liver biopsy might be necessary for patients infected with HCV genotype 1 or 4, if there are discordant results from TE and biomarker assays, before a treatment decision is made. In making the decision to re-treat a patient, a liver biopsy might be required to identify factors that impaired the original response to therapy, such as non-alcoholic steato-hepatitis, if a liver biopsy was not previously performed.
However, with the development of direct-acting agents,98, 99 particularly with next-generation direct-acting agents or interferon-free regimens, which produce higher rates of sustained virologic response,100, 101 discriminating between fibrosis stages F0-F1 and ≥F2 might not be relevant in determining treatment indications.
Noninvasive tests for fibrosis have been less well-incorporated into management of HBV than HCV because there have been fewer studies and because liver inflammation and HBV replication confound interpretation of test results. In treatment-naïve patients, noninvasive tests could be used for patients with levels of ALT <2-fold the upper limit of normal and levels of HBV DNA >20,000 IU/mL (for HBeAg-positive patients) or >2000 IU/mL (for HBeAg-negative patients).1, 2 Results from studies combining TE and biomarker assays remain too preliminary to make recommendations. Some researchers have proposed diagnostic algorithms that use dual TE cut-offs, for positive and negative prediction of significant fibrosis.102 Obviously, the applicability of this dual cut-off strategy is determined by the setting and the probability that patients have significant fibrosis. For instance, a cut-off value <6.2 kPa could rule out significant fibrosis in virtually all patients (87%) with a low probability for significant fibrosis, such as inactive carriers. Alternatively, a cut-off value of >9.4 kPa accurately predicted fibrosis (92%) in patients with a higher probability of significant fibrosis, such as middle-aged, HBeAg-negative patients with persistently abnormal levels of ALT. The remaining patients should still undergo liver biopsy analysis.1
Monitoring Treatment Response
A major advantage of noninvasive methods, compared with liver biopsy, is that the noninvasive assays can be easily repeated over time as patients are followed. For instance, in patients already receiving antiviral therapy, TE and biomarker assays could be used to monitor response to treatment and to evaluate fibrosis regression. Significant histologic improvements have been documented in studies of paired liver biopsies from patients with chronic hepatitis C who achieved sustained viral eradication103, 104 and patients with chronic hepatitis B who received long-term antiviral therapy.105, 106Several recent studies reported a significant decrease in liver stiffness and biomarkers values, compared with baseline values, in patients with HCV who achieved sustained viral eradication,107, 108, 109, 110, 111, 112, 113 as well as in HBV-infected patients treated with analogues.114, 115, 116, 117, 118, 119, 120, 121
Despite these encouraging results, following the progress of treated patients with TE or biomarkers can be confounded by changing levels of ALT and inflammation. Some tests for serum biomarkers include parameters that could be affected by the resolution of inflammation (including measurements of ALT and AST). Similarly, a decrease in liver stiffness could result from reductions in inflammatory activity, rather than fibrosis. However, in the only study113 that assessed liver stiffness kinetics at multiple time points during therapy (weeks 4 and 12) and afterward (week 24), liver stiffness decreased significantly with treatment among patients who did and did not achieve sustain viral eradication; stiffness continued to decrease significantly after the end of treatment only in patients with sustained viral eradication. Studies with paired liver biopsies are needed to determine whether TE and serum biomarker assays can be used to follow treatment response but are unlikely to be conducted for ethical reasons. Assessments of liver stiffness within 6 months after the end of therapy in patients with HCV infection are probably not clinically meaningful. For patients with HBV infection, serial measurements of liver stiffness should be performed after ALT levels have normalized, over the long-term course of treatment. In patients with cirrhosis, post-treatment assessments of liver stiffness should not substitute for the recommended, periodic surveillance for HCC, using ultrasound examination and measurement of α-fetoprotein levels.122
Monitoring Disease Progression
Noninvasive methods can be used to identify patients with cirrhosis who are at risk of disease progression. Compensated cirrhosis is classified as that without varices (stage 1) or with varices (stage 2). Compensated cirrhosis could be further subdivided, as that with no portal hypertension, portal hypertension that is not clinically significant (hepatic venous pressure gradient [HVPG], <10 mm Hg), or clinically significant portal hypertension (HVPG, ≥10 mm Hg).123
Measurements of liver stiffness might be used to assess clinical outcomes because they correlate with the severity of liver disease,124 according to a retrospective study conducted in a single center. This study provided the first "proof of concept" that liver stiffness is a prognostic factor for patients with cirrhosis. Furthermore, liver stiffness correlates with portal pressure (based on the HVPG),125, 126, 127, 128 which accurately predicts clinical events.129 Interestingly, there was a high degree of correlation between liver stiffness and only HVPG values below 10-12 mm Hg.126 This indicates that, beyond a certain degree of portal pressure (above 10-12 mm Hg), development of portal hypertension is at least partially independent from the simple accumulation of fibrillar extracellular matrix, which is responsible for the increase in liver stiffness. Conversely, repeated measurements of liver stiffness, over time, might be made during the first year after liver transplantation to identify patients with early-stage recurrence of severe hepatitis C recurrence and reduce the need for follow-up liver biopsies.125, 130, 131
Liver stiffness values have also been correlated with the presence of esophageal varices. However, the diagnostic accuracy of TE (specificity below 60%) is too low for identification of patients with esophageal varices in clinical practice.132 When biomarkers were compared, in a large-scale multicenter study of factors to predict which patients would develop high-risk esophageal varices (large esophageal varices, those with red signs, or decompensated cirrhosis), the combination of Lok index and Forns index had the best diagnostic performance, avoiding endoscopy in around one-third of patients.133 There might be also a role for other noninvasive models combining simple biomarkers such as AST/ALT ratio or platelet count.134When compared with serum biomarkers, TE did not perform better for the detection of esophageal varices and large esophageal varices.88 However, a strategy combining TE with spleen diameter and platelet count (referred as LSPS for LSM-Spleen diameter to platelet ratio score) has been shown to increase diagnostic accuracy for detecting high-risk esophageal varices in patients with HBV-related cirrhosis.135 Interestingly, Liver Stiffness Measurement-Spleen Diameter and Platelet Count Score (LSPS) appeared as a reliable predictor of esophageal varices bleeding risk in these patients.136 These findings are consistent with those of a study that reported that liver stiffness values can be as effective as HVPG measurements in predicting which patients will develop clinical decompensation and portal hypertension-related complications.137 For instance, at a cut-off of 21.1 kPa, TE had a 100% negative predictive value for the occurrence of portal hypertension-related complications; if these results are confirmed, TE could be used as a prognostic tool.138
In summary, TE results can identify patients most likely to develop clinically significant portal hypertension but are not sufficient to identify patients with esophageal varices, in clinical practice, or replace endoscopy analysis of cirrhotic patients.132 Given its likely prognostic value for patients with cirrhosis, TE could be used to rapidly discriminate among patients at different stages of progression of compensated cirrhosis and place them in different risk categories.139
Determining Prognosis
Noninvasive methods can also be used in determining prognosis. Large, prospective cohort studies in Asia of patients with hepatitis B or C correlated liver stiffness values with HCC occurrence.140, 141, 142 Among 866 Japanese patients with HCV infection, the cumulative incidence of HCC within 3 years was as high as 38.5%, among subjects with baseline liver stiffness values >25 kPa, compared with 0.4% among subjects with values ≤10 kPa.140 Although measurements of liver stiffness could be used to identify patients at risk of developing HCC, more data are needed before they could be integrated into an HCC surveillance program.
Prognosis of patients with chronic liver disease related to viral hepatitis or other causes can be determined using TE137, 143 and assays for serum biomarkers such as FibroTest,94, 144, 145 ELF (iQur Ltd, Southsampton, United Kingdom),146, 147 APRI, and FIB-4,148 as well as for models based on standard laboratory tests.149, 150 A recent French study143 compared the ability of different noninvasive methods (TE, FibroTest, APRI, and FIB-4) to predict survival and liver-related death of 1457 patients with HCV infection; liver stiffness values and results from the FibroTest had the highest 5-year predictive values, which did not change after adjustment for treatment response, patient age, or estimates of necroinflammatory grade. The potential of noninvasive methods for predicting clinical outcomes seems to be greater than that of liver biopsy; probably the noninvasive tests measure ongoing pathophysiologic processes and functions that a biopsy cannot. Additional advantages of assays for serum biomarkers and TE over histologic scoring systems are that they provide a range of continuous values, instead of a limited number of categories.151
Future Directions
Significant progress has been made over the past decade in noninvasive assessment of liver disease in patients with hepatitis B or C, but there is no perfect method. On the one hand, there is increasing awareness that liver biopsy is an imperfect standard. On the other hand, an increasing number of noninvasive methods are available: TE, FibroTest, and APRI are the most widely used and validated worldwide. The introduction of these methods several years ago in France for the management of patients with hepatitis C in routine practice significantly reduced the need for liver biopsy,152 and this trend has since been observed in most countries where these methods have been implemented. However, noninvasive methods will reduce, but not completely end, the need for liver biopsy.153 Liver biopsies and noninvasive methods should be used as an integrated system to allow more efficient evaluation of patients with hepatitis B or C.52
It is important to investigate the prognostic value of noninvasive methods of fibrosis detection, particularly TE, for patients with cirrhosis; these tests could be used to better classify patients with cirrhosis and assign them to different categories of risk for clinical outcomes. TE has limitations and is challenged by other technologies to measure liver stiffness, such as ARFI and MR elastography, whose place in clinical practice remains to be defined. Other promising techniques, such as supersonic shear imaging154, 155 or measurements of spleen stiffness,156 could also become available and deserve further evaluation. It has been proposed that noninvasive methods be used to screen the general population for cirrhosis.157, 158, 159 However, this approach is not likely to be cost-effective, given the low prevalence of cirrhosis in the general population.
|
|
| |
| |
|
|
|