| |
Estimates on HCV disease burden worldwide - filling the gaps
|
| |
| |
Download the PDF here
"The concept of using the new DAAs as 'treatment as prevention' is appealing and may become important in specific circumstances, for example, with injection drug users and in prisons. The current analysis did not take into consideration the impact of treatment as prevention."
Eliminating HCV " Test & Treat" - HCV is a Disease of the Marginalized: homeless, IDUs, immigrants, African-Americans, Latinos........
http://www.natap.org/2014/HCV/093014_01.htm
HCV is A Disease of the Marginalized- IDUs, homeless:
http://www.natap.org/2014/HCV/052814_02.htm
Journal of Viral Hepatitis
Jan 2015
H. Wedemeyer,1 G. J. Dore2 and J. W. Ward3 1Hannover Centre for Internal Medicine, Hannover, Germany; 2Kirby
Institute, University of New South Wales, Sydney, NSW, Australia; and 3Division of Viral Hepatitis, Centers for Disease Control and Prevention,
Atlanta, GA, USA
Summary
Hepatitis C is caused by infection with the hepatitis C virus (HCV) and represents a major global health burden. Persistent HCV infection can lead to progressive liver disease with the development of liver cirrhosis and hepatocellular carcinoma, possibly accounting for up to 0.5 million deaths every year. Treatment of HCV infection is undergoing a profound and radical change. As new treatments are extremely safe and effective, there are virtually no medical reasons to withhold therapy. Yet, the new therapies are expensive. As resources are limited, solid data to estimate the disease burden caused by HCV are urgently needed. Epidemiology data and disease burden analyses for 16 countries are presented. For almost all countries, the peak of HCV-related cirrhosis, hepatocellular carcinoma and liver-related death is a decade or more away. However, a surprising heterogeneity in country-specific HCV-associated disease burden exists. Also, HCV diagnosis and treatment uptake varied markedly between countries. A consistent finding was that a reduction of HCV liver-related mortality is dependent on access to therapy. Increasing efficacy of therapy alone with a constant numbers of treatments will not have a major impact on the HCV-related disease burden. The data presented here should inform public health policy and help drive advocacy for enhanced strategic investment and action. HCV kills patients, and the disease burden will continue to rise in most countries unless action is taken soon. Chronic HCV is a curable infection and a reversible liver disease. Fortunately, the tools to eliminate HCV are now available.
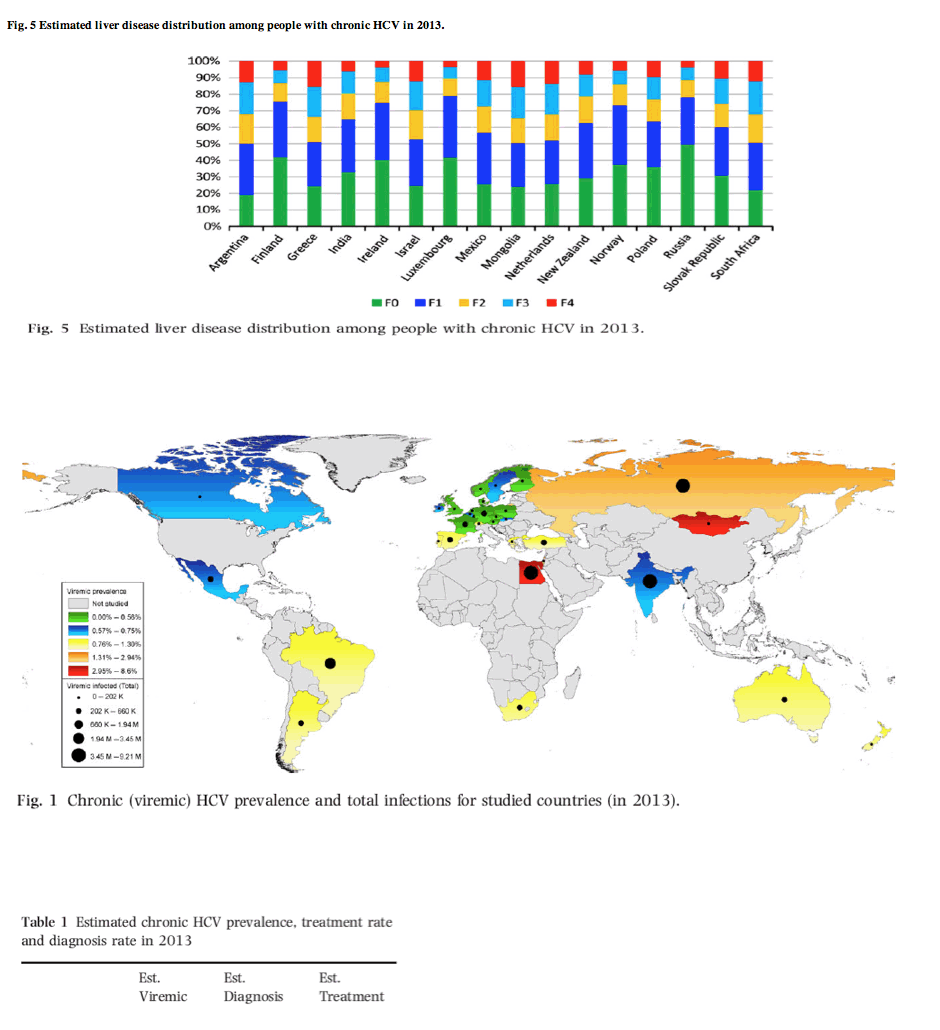
Fig. 5 Estimated liver disease distribution among people with chronic HCV in 2013.
Introduction
Hepatitis C is caused by infection with the hepatitis C virus (HCV) and represents a major global health burden. Persistent HCV infection can lead to progressive liver disease with the development of liver cirrhosis and hepatocellular carcinoma, possibly accounting for up to 0.5 million deaths every year [1]. The true number of HCV infections worldwide is unknown, but a recent estimate, accounting for various confounders including reduced prevalence among children, suggested that between 64 and 103 million individuals have chronic HCV infection [2].
Treatment of HCV infection is undergoing a profound and radical change. For more than two decades, the administration of interferon alpha has been the basis of all HCV therapies. Depending on the HCV genotype, viral load, stage of liver disease and distinct host genetic polymorphisms close to the interferon lambda gene, between 30% and 90% of patients responded to the previous standard of care with PEG-interferon alfa in combination with ribavirin [3]. However, many patients could not be treated due to side effects and contraindications. Still, if therapy was successful, patients who achieved a sustained viral response (SVR) had reduced liver-specific mortality and improved overall survival [4]. The first direct acting antiviral (DAA) drugs against HCV were introduced in 2011. These first HCV protease inhibitors, boceprevir and telaprevir, were used in combination with interferon alfa and ribavirin and were associated with increased toxicity and treatment complexity [5]. Only 3 years after approval, both first generation protease inhibitors are no longer recommended in many countries [6, 7].
Since 2013-2014, interferon-free therapy has become a reality. By early 2015, the Food and Drug Administration (FDA) and the European Medical Agency (EMA) should have approved 6-7 novel DAAs including the nucleotide polymerase inhibitor sofosbuvir, a 2nd generation protease inhibitor (simeprevir), the first HCV-NS5A inhibitor (daclatasvir; EMA only), a single-tablet combination of sofosbuvir with the NS5A inhibitor ledipasvir, and the '3D regimen' of paritaprevir (ritonavir-boosted), ombitasvir and dasabuvir. SVR rates in pivotal phase 2 and 3 trials have been between 92% and 100% even in pretreated HCV genotype 1 infected patients [8]. In addition, more drugs are on the horizon exploring treatments as short as 4 weeks for chronic HCV infection. These remarkable advances in HCV therapeutic options are rather unique in modern medicine. For the first time, a chronic disease can be cured in more than 90% of patients with just 3 months of therapy!
The amazing advance in HCV therapy, however, and rather ironically, also represents a challenge to many health systems across the globe. As new treatments are extremely safe and effective, there are virtually no medical reasons to withhold therapy. Yet, the new therapies are expensive. As resources are limited, solid data to estimate the disease burden caused by HCV are urgently needed. Even more importantly, potential effects of increased efficacy and higher treatment uptakes will help stakeholders negotiate prices and prioritize restricted funds. These estimates have to account for distinct characteristics of patient populations in individual countries. Moreover, treatment strategies, guidelines and reimbursement differ largely between countries. Even within the European Union, completely different scenarios have to be considered in Northern, Central, Eastern and Southern European health systems [9, 10].
In May 2014, a supplement was published in the Journal of Viral Hepatitis presenting data on the historical epidemiology, the disease burden and strategies to manage HCV for 16 different countries [11-13]. These papers offered models for Australia, Egypt, Brazil and 13 European countries including England, France, Germany, Spain and Turkey. It was striking to see that for almost all countries, the peak of HCV-related cirrhosis, hepatocellular carcinoma and liver-related death is a decade or more away. However, a surprising heterogeneity in country-specific HCV-associated disease burden became evident. Also, HCV diagnosis and treatment uptake varied markedly between countries.
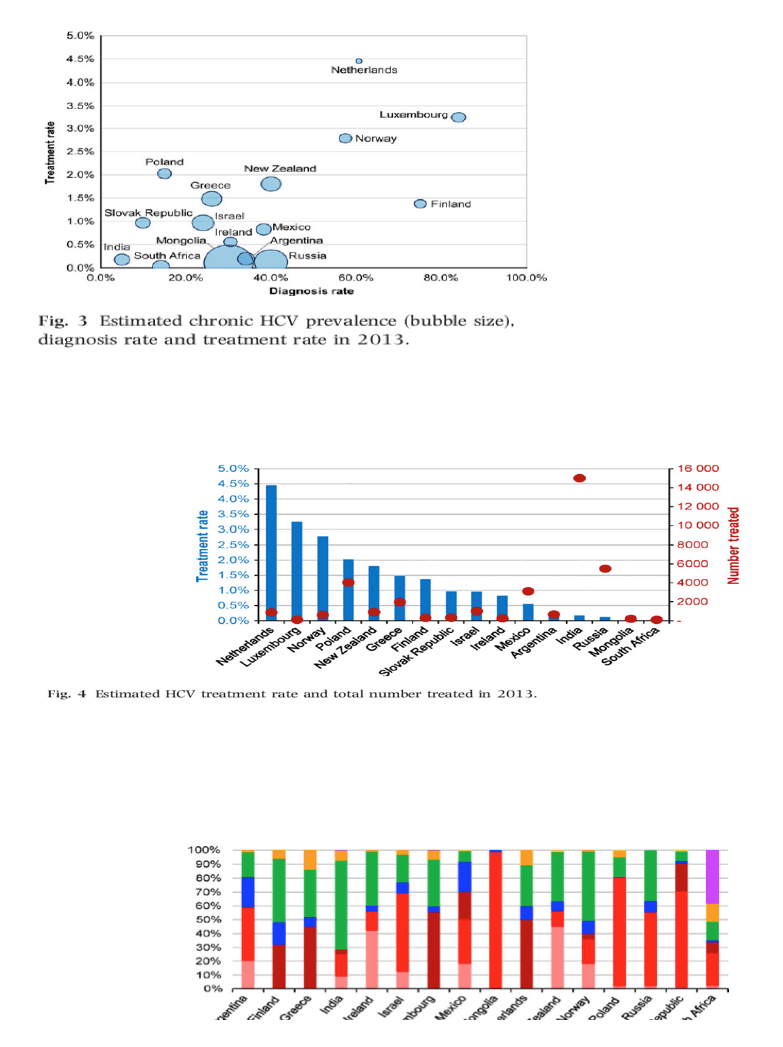
In this supplement, data for an additional 16 countries are presented using the same model as in the previous papers [14-16]. Of note, the new papers include more European countries but also analysis for Argentina, India, Israel, Mexico, New Zealand, Russia and South Africa. Figure 1 shows the chronic (viremic) HCV prevalence and the number of infections for all 32 countries studied. In countries with a large population (e.g. India), an HCV prevalence of <1% still resulted in a large number of HCV infections. There is significant variability in genotype distribution among countries reported in this supplement (Fig. 2) with South Africa having a large genotype 5 population while the HCV infections in India are predominately genotype 3. There is also a large variation in chronic HCV prevalence, diagnosis rate and treatment rate (Fig. 3, Table 1).Western European countries typically reported a higher diagnosis and treatment rate. Treatment rate, defined as the number of patients treated in a single year divided by the total chronic HCV infections, is shown in more details in Fig. 4. India and Russia, which have a low treatment rate, in fact treat more patients with HCV (annually) than other countries studied. However, due to the very high number of chronic infections in each country, the treatment rate remains very low. Countries with a young infected population (Russia, Ireland, Finland and Luxembourg), typically associated with injection drug use acquired infections, were forecasted to have a higher percentage of their population in the early stages of fibrosis (F0-F1) as shown in Fig. 5. On the other hand, countries with an older infected population (Argentina, Greece, Mongolia and South Africa), typically associated with nosocomially acquired infections, had HCV-infected populations with more advanced stages of fibrosis (F3-F4). A consistent finding was that a reduction of HCV liver-related mortality is dependent on access to therapy [16]. Increasing efficacy of therapy alone with a constant numbers of treatments will not have a major impact on the HCV-related disease burden - with no major differences between countries such as Poland, South Africa, Argentina, New Zealand or India.
The outcomes from each country are informative and important, but limitations of the approach and the model need to be considered [17, 18]. As robust surveillance data were sometimes lacking, some input estimates relied in part on expert panel consensus and not on unbiased published data. Moreover, the model was not 'dynamic', thus, re-infections and secondary infections were assumed to remain constant. The concept of using the new DAAs as 'treatment as prevention' is appealing and may become important in specific circumstances, for example, with injection drug users and in prisons. The current analysis did not take into consideration the impact of treatment as prevention.
Finally, cost effectiveness was not considered as part of this analysis. This is an important topic that impacts access to care. Due to the number of countries being analysed and the difficulty in collecting consistent cost data across all countries, the impact of drug cost was not taken into consideration when assessing different strategies. The focus of the analysis was on disease burden.
The data presented here should inform public health policy and help drive advocacy for enhanced strategic investment and action. HCV kills patients, and the disease burden will continue to rise in most countries unless action is taken soon. Chronic HCV is a curable infection and a reversible liver disease. Fortunately, the tools to eliminate HCV are now available.
|
|
| |
| |
|
|
|