| |
Incidence and prevalence of hepatitis c virus infection among
persons who inject drugs in New York City: 2006-2013...68% HCV Prevalence
|
| |
| |
Download the PDF here
......."HCV prevalence among these 1535 PWID was 68.2%......we did not observe any significant decrease in HCV prevalence or incidence......HIV prevalence among PWID recruited between 2006 and 2013 was 11.9% (183 of 1535; 95% CI: 10-14%). There was a statistically significant decrease in HIV prevalence over the 2006-2013 study period (from 17% to 4.3%)"
"These data strongly suggest an ongoing, high prevalence HCV epidemic among PWID in NYC......HCV is more readily transmitted by non-sterile injection practices than is HIV.......These data demonstrate that the HCV prevalence among PWID and the estimated HCV incidence among new injectors both remain high in NYC. During the 2006-2013 study period of combined prevention programming HIV prevalence among PWID decreased significantly, yet current prevention strategies have not led to comparable reductions in HCV prevalence or estimated incidence among new injectors......Modeling suggests that a significant scale up of HCV treatment would be necessary to reduce HCV prevalence by three-quarters within 15 years (Martin et al., 2013). While specific data for NYC are not available, there have continued be very significant gaps in the HCV continuum of care for PWID in most regions including NYC, with few individuals being linked to and engaged in HCV treatment......The more widespread implementation of effective linkage to care models will be needed for HCV treatment as prevention to impact the ongoing HCV epidemic among PWID in NYC......In conclusion, there continues to be a high prevalence of an HCV epidemic among PWID in NYC. New injectors in NYC remain at high risk for HCV. More potent combined prevention, including significant scale up of some combination of MAT, sterile drug injection equipment access, and HCV treatment is urgently needed to control the HCV epidemic among PWID in NYC.
HCV prevalence among these 1535 PWID was 68.2% (1047 of 1535; 95% CI: 66-70%).........HIV prevalence among PWID recruited between 2006 and 2013 was 11.9% (183 of 1535; 95% CI: 10-14%). There was a statistically significant decrease in HIV prevalence over the 2006-2013 study period (from 17% to 4.3%; p-value for trend: <0.0001).
The expanded combined prevention programming in NYC during the 2006-2013 period included multiple interventions with the potential to reduce the incidence and prevalence of both HIV and HCV (e.g., MAT, SEP, ESAP, and more effective antiviral treatment options) as well as additional programs with the potential to impact HIV transmission (e.g., the NYC Condom program and expanded use of ART among PWID and hence, HIV TasP; Des Jarlais et al., 2015). As previously reported, HIV prevalence and incidence declined substantially among PWID during this period.
In contrast, while the expanded combined prevention programming in NYC during this time period did include several interventions with the potential to impact HCV transmission (MAT, SEP, ESAP, as well as significantly improved HCV treatment options), we did not observe any significant decrease in HCV prevalence or incidence. During this period, PWID in general, and new injectors in particular, had incomplete access to or engagement in MAT (there was no additional expansion of MAT during this period and most PWID were not consistently in MAT) and the majority had incomplete sterile needle and syringe coverage (whether through SEP and/or ESAP), many of the new injectors engaged in some form of non-sterile injection, and the overwhelming majority were not receiving MAT (in the 6 months prior to entry into the study), even in this cohort recruited in drug treatment settings. HCV is more readily transmitted by non-sterile injection practices than is HIV (Alter, 2006, Hagan, 2011); hence, the impact of any degree of sterile needle, syringe, and drug preparation equipment ("drug injection equipment") access is likely to have less of an effect on HCV transmission than on HIV transmission. Therefore, degrees of MAT and sterile drug injection equipment access sufficient to contribute to effective HIV combination prevention programming may be insufficient for effective HCV prevention programming.
Overall, the estimated incidence of HCV among new injectors during the study period was 19.5/100 PYO (95% CI: 17-23). This estimated HCV incidence among new injectors did not differ from the estimated incidence in 2000-2001 of 18/100 PYO (95% CI: 14-23/100 PYO). There was no significant difference in estimated HCV incidence among new injectors in each year of study recruitment (Table 4).
Of the 347 new injectors, 73% reported injecting at least once daily in the past 6 months; the proportion doing so did not change over the 2006-2013 study period (test for trend over time p = 0.31). Sixty eight percent reported obtaining none of their needles and syringes from an SEP in the past 6 months; this proportion did not change over the study period (p = 0.22). Twenty seven percent reported sharing a cooker at least once in the past 6 months; this proportion did not change over the study period (p = 0.65). Twenty five percent reported at least 1 instance of injection with a syringe previously used by someone else in the past 6 months; this proportion did not change over the study period (p = 0.5).
Ninety-one percent (N = 331) of the new injectors were recruited from detoxification, only 15% of whom had received any methadone maintenance in the past month; this proportion did not change over the study period (p = 0.42)."
----------------------
Incidence and prevalence of hepatitis c virus infection among persons who inject drugs in New York City: 2006-2013
Drug and Alcohol Dependence July 2015
Ashly E.Jordan a,b, DonC. Des Jarlais b,c, Kamyar Arasteh b,c, Courtney McKnight b,c, Denis Nash d, David C. Perlman b,e
Abstract
Background
Hepatitis C virus infection is a source of significant preventable morbidity and mortality among persons who inject drugs (PWID). We sought to assess trends in hepatitis C virus (HCV) infection among PWID from 2006 to 2013 in New York City (NYC).
Methods
Annual cross-sectional surveys of PWID entering a large drug abuse treatment program were performed. Risk behavior questionnaires were administered, and HIV and HCV testing were conducted. Comparisons were made with prior prevalence and incidence estimates in 1990-1991 and 2000-2001 reflecting different periods of combined prevention and treatment efforts.
Results
HCV prevalence among PWID (N: 1535) was 67% (95% CI: 66-70%) during the study period, and was not significantly different from that observed in 2000-2001. The estimated HCV incidence among new injectors (persons injecting for ²6 years) during 2006-2013 was 19.5/100 PYO (95% CI: 17-23) and did not differ from that observed in 2000-2001 (18/100 PYO, 95% CI: 14-23/100).
Conclusions
Despite the expansion of combined prevention programming between 2000-2001 and 2006-2013, HCV prevalence remained high. Estimated HCV incidence among new injectors also remained high, and not significantly lower than in 2000-2001, indicating that expanded combined prevention efforts are needed to control the HCV epidemic among PWID in NYC.
Introduction
Hepatitis C virus (HCV) infection is a major source of preventable morbidity and mortality among persons who inject drugs (PWID), among whom HCV is hyperendemic (Hagan, 2011, Ly et al., 2012). HCV is readily transmissible via non-sterile injections (Hagan, 2011). Its transmissibility, combined with a high population prevalence among PWID (estimated at 43-67% among lifetime PWID; Armstrong et al., 2006, Lansky et al., 2014, World Health Organization, 2014), and a high prevalence of drug injection behaviors that facilitate transmission (e.g., 30% rates of receptive syringe sharing; Des Jarlais et al., 2010), leads to situations of high annual incidence of HCV infection (Backmund et al., 2005, Hagan, 2011, Ly et al., 2012, Wiessing et al., 2014).
Nonsterile iatrogenic nosocomial injection-related transmission, and other healthcare-related exposures, have been greatly reduced in settings where infection control practices and screening of blood and organ products have been implemented (Averhoff et al., 2012, Backmund et al., 2005); in these settings nonsterile illicit drug injection is the predominant mode of HCV transmission (Alter, 2011b). HCV epidemics became established in populations of PWID in the 1960s to 1990s, preceding and exceeding the epidemic of HIV and leading to high prevalence epidemics in many cities (Alter, 2011a, Nelson et al., 2011). In New York City (NYC) in the 1990s, approximately 80-90% of PWID were HCV infected and 50% were HIV infected (Des Jarlais et al., 2005b).
In the period from the mid-1990s to the mid-2000s, a number of interventions were developed and implemented in an attempt to prevent blood borne pathogen transmission via non-sterile injections (Des Jarlais et al., 2009a, Des Jarlais et al., 2005b, Hagan et al., 2011). We have previously shown that the expansion of combined prevention programming with continued medication assisted treatment (MAT) of opioid dependence (e.g., methadone maintenance), expanded needle and syringe exchange programs (SEPs), and the introduction of anti-retroviral therapy (ART) were temporally associated with very significant reductions in HIV prevalence and incidence among PWIDs in NYC (Des Jarlais et al., 2005a). During the same period of initial combined prevention, HCV prevalence decreased from 91% to 62% among PWID, while the incidence of HCV infection among new injectors (persons who began injecting within the prior 6 years) remained quite high (estimated as 18/100 person years of observation (PYO) in 2000-2001; Des Jarlais et al., 2010, Des Jarlais et al., 2005b).
Since that time there has been a continued expansion of combined HIV and HCV prevention efforts, which has included evolving and improving treatment for HCV, as well of efforts at HCV education and testing, and changes in community level awareness of HCV (Gow and Mutimer, 2001, Heller and Paone, 2011, Tesoriero et al., 2009); however, there was not an expansion of HCV treatment among PWID comparable to the expansion of ART treatment of HIV among PWID (Grebely and Dore, 2014, Des Jarlais et al., 2015, Wiessing et al., 2014). Concurrent with these expanded combined prevention efforts, there was a continued decline in HIV incidence and prevalence among PWID (Des Jarlais et al., 2010, Des Jarlais et al., 2015), but the impact on HCV incidence and prevalence among PWID in NYC has not been characterized.
We now extend previous observations to examine HCV prevalence and incidence among PWID in NYC in the period 2006-2013 and compare these with data from1990-1991 and 2000-2001 collected with the same methods (Des Jarlais et al., 2005b). We hypothesized that due to the higher infectivity of HCV compared to HIV, and to the less extensive implementation of antiviral treatment for HCV than for HIV among PWID, that the impact of combined prevention programming on HCV incidence and prevalence might be less than the impact observed on HIV incidence and prevalence. The specific objectives include (1) estimating HCV prevalence among PWID, (2) estimating HCV incidence among new injectors in NYC and (3) comparing HCV prevalence, and estimated incidence among new injectors, who began injecting during different time periods reflecting the availability of different combined prevention public health efforts.
Results
Between 2006 and 2013 a total of 4100 participants were recruited into the "Risk Factors" study. The proportion reporting a history of everdrug injection (i.e., current or former drug injection) increased significantly over the 2006-2013 study period from 35.6% (224 of 630) to 51.8% (248 of 478; p-value for trend: <0.0001) for a total of 1774 PWID recruited during this study period. Two hundred and eleven (11.9%) did not have HIV data available, 87% of whom did not have HCV data available; 213 (12%) PWID did not have HCV data available, 88% of whom did not have HIV data available. Complete HIV and HCV data were available for 1535 of 1774 PWID.
Table 1 presents characteristics of these 1535 PWID study participants. The mean age of initiation of drug injection was 24.4 years (SD: 8.3). Approximately half (N: 766; 50.5%) reported initiating drug injection in 1995 or after (see Table 1). HIV prevalence among PWID recruited between 2006 and 2013 was 11.9% (183 of 1535; 95% CI: 10-14%). There was a statistically significant decrease in HIV prevalence over the 2006-2013 study period (from 17% to 4.3%; p-value for trend: <0.0001).
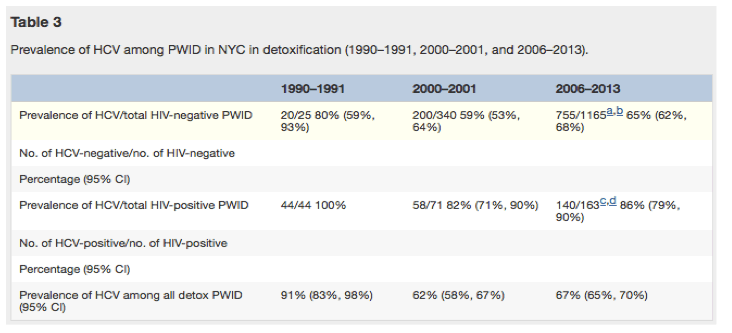
There were modest, but not significant, decreases in HCV prevalence among HIV-negative PWID recruited at detoxification between 2006 and 2013 (p-value: 0.09) and among HIV-negative PWID recruited at MMTP (2011-2013) (p-value: 0.19). There was no change in HCV prevalence among HIV-positive PWID recruited at detoxification between 2006 and 2013 (p-value: 0.85) or among HIV-positive PWID recruited at MMTP between 2011 and 2013 (p-value: 0.52) (see Table 2). HCV prevalence among all PWID recruited at the detoxification program significantly decreased over the 2006-2013 study period from 76% to 59% (p-value for trend: 0.009); there was a concurrent significant decrease in the proportion of PWID recruited at the detoxification program who were HIV-infected over the same eight year period (test for trend p = 0.001; Table 2) from 16.9% in 2006 to 3.4% in 2013.
Table 3 depicts the HCV prevalence among all PWID, HIV-negative PWID, and HIV-positive PWID recruited at detoxification between 2006 and 2013 and compares them with the HCV prevalence previously found in these groups among those recruited in 1990-1991 and 2000-2001. While HCV prevalence decreased among all PWID between 1990-1991 and 2000-2001 as previously reported,(Des Jarlais et al., 2005b) the HCV prevalences among all PWID, HIV-negative PWID and HIV-positive PWID in 2006-2013 do not differ from those identified in 2000-2001.
There were 395 newly initiated PWID recruited between 2006 and 2013, of whom 347 were current PWID. Of these 347 new injectors, 141 were HCV seropositive (41%; 95% CI: 36-46%). The estimated incidence of HCV among these new injectors in each recruitment year is depicted in Table 4. Overall, the estimated incidence of HCV among new injectors during the study period was 19.5/100 PYO (95% CI: 17-23). This estimated HCV incidence among new injectors did not differ from the estimated incidence in 2000-2001 of 18/100 PYO (95% CI: 14-23/100 PYO). There was no significant difference in estimated HCV incidence among new injectors in each year of study recruitment (Table 4).
Discussion
These data demonstrate that the HCV prevalence among PWID and the estimated HCV incidence among new injectors both remain high in NYC. During the 2006-2013 study period of combined prevention programming HIV prevalence among PWID decreased significantly, yet current prevention strategies have not led to comparable reductions in HCV prevalence or estimated incidence among new injectors.
These data strongly suggest an ongoing, high prevalence HCV epidemic among PWID in NYC. While these data are based on HCV antibody data, there is no reason to suspect any change in the proportion of those clearing HCV infection and hence the persistent high HCV prevalence also suggests a continued high HCV community viral load. The estimated incidence of HCV among new injectors remained high (19.5/100 PYO) and was not significantly different than that we observed in 2000-2001 (18/100 PYO, 95% CI = 14-23) (Des Jarlais et al., 2005b). These incidence estimates are consistent with recent estimates among young current PWID recruited in San Francisco in 2000-2013 (Tsui et al., 2014). However, reductions in HCV incidence have been observed in some other settings (e.g., Australia and Vancouver, Canada), in association with expansions of SEP and MAT prevention programming, reductions in syringe borrowing, but in at least in instance, in association with increases in crack cocaine use (Grebely and Dore, 2014, Iversen et al., 2013, White et al., 2014).
The expanded combined prevention programming in NYC during the 2006-2013 period included multiple interventions with the potential to reduce the incidence and prevalence of both HIV and HCV (e.g., MAT, SEP, ESAP, and more effective antiviral treatment options) as well as additional programs with the potential to impact HIV transmission (e.g., the NYC Condom program and expanded use of ART among PWID and hence, HIV TasP; Des Jarlais et al., 2015). As previously reported, HIV prevalence and incidence declined substantially among PWID during this period (Des Jarlais et al., 2009a, Des Jarlais et al., 2010).
In contrast, while the expanded combined prevention programming in NYC during this time period did include several interventions with the potential to impact HCV transmission (MAT, SEP, ESAP, as well as significantly improved HCV treatment options), we did not observe any significant decrease in HCV prevalence or incidence. During this period, PWID in general, and new injectors in particular, had incomplete access to or engagement in MAT (there was no additional expansion of MAT during this period and most PWID were not consistently in MAT) and the majority had incomplete sterile needle and syringe coverage (whether through SEP and/or ESAP), many of the new injectors engaged in some form of non-sterile injection, and the overwhelming majority were not receiving MAT (in the 6 months prior to entry into the study), even in this cohort recruited in drug treatment settings. HCV is more readily transmitted by non-sterile injection practices than is HIV (Alter, 2006, Hagan, 2011); hence, the impact of any degree of sterile needle, syringe, and drug preparation equipment ("drug injection equipment") access is likely to have less of an effect on HCV transmission than on HIV transmission. Therefore, degrees of MAT and sterile drug injection equipment access sufficient to contribute to effective HIV combination prevention programming may be insufficient for effective HCV prevention programming.
Another important difference between the expanded combined prevention programming implemented during this period with respect to HCV and to HIV transmission is the extent of antiviral treatment coverage implemented for the two infections. While the NIH consensus statement of 2002 suggested that PWID should be offered HCV treatment on a case by case basis (National Institutes of Health, 2002), during this period, very few PWID initiated or completed HCV treatment (Aspinall et al., 2013, Linas et al., 2014, Mehta et al., 2006). While data regarding the potential for HCV treatment to function as HCV treatment as prevention are unclear, what is clear is that there is no potential for it to do so if HCV-infected PWID are not treated. Modeling suggests that a significant scale up of HCV treatment would be necessary to reduce HCV prevalence by three-quarters within 15 years (Martin et al., 2013). While specific data for NYC are not available, there have continued be very significant gaps in the HCV continuum of care for PWID in most regions including NYC, with few individuals being linked to and engaged in HCV treatment; recent estimates are that only 1-9.5% of HCV infected PWID initiate treatment (Grebely and Dore, 2014, Wiessing et al., 2014). The more widespread implementation of effective linkage to care models will be needed for HCV treatment as prevention to impact the ongoing HCV epidemic among PWID in NYC (Jordan et al., 2012, Masson et al., 2013). While our data cannot quantify the contribution of the impact of gaps in MAT coverage, sterile drug injection equipment access or HCV treatment to the failure of combined prevention programming to reduce HCV incidence during this period, the data clearly demonstrate that more potent combined HCV prevention programs are needed.
Modeling has suggested that the ability of HCV treatment as prevention to impact the HCV epidemic among PWID, and to do so cost-effectively, is reduced when the HCV prevalence is approximately 60% (Martin et al., 2012), as was found among PWID in NYC in our study. While estimates of approximate levels of MAT, SEP and TasP coverage that may be required to reduce HCV incidence among PWID in NYC are needed, these findings, combined with the identified gaps in access to sterile syringes, suggests that HCV control among PWID in NYC is likely to require some expansion of MAT and/or sterile drug injection equipment access in concert with treatment as prevention.
This study has limitations. While participants were selected randomly from among detoxification and MMTP entrants, there is a potential for selection bias with respect to representativeness of PWID in NYC; however, neither the number of detoxification or MMTP treatment slots, nor their admission criteria, changed significantly during the study period. Measures of HCV prevalence relied on HCV antibody testing; while HCV viral loads were not done, there is no substantive reason to suspect that rates of HCV clearance would differ from those generally observed, or that they would change over the periods of study and comparison. As the study is a series of annual cross-sectional surveys, direct data on HCV seroconversion were not available. The incidence estimates relied on several key assumptions. PWID were assumed to have been HCV negative when they started injecting; were this not the case, estimates of HCV incidence might have been lower. If there were greater differential loss of HCV positive PWID compared with HCV negative PWID in the study population, estimates of HCV incidence might have been higher. However, the assumptions made for incidence estimates in both the 2000-2001 and 2006-2013 time periods were the same, and the incidence estimates we found are consistent with other recent data from San Francisco (Tsui et al., 2014). Both to allow direct comparison with our previous study and to afford more robust incidence estimates, we defined recent injection initiation as being within the past 6 years; however, comparative data on incidence during the first several years of injection would be valuable.
Individuals who were included in the study more than once would have been included in prevalence estimates in each year they were included; this in fact provides an accurate measure of HCV prevalence. Since such persons represent <3% of the cohort, their inclusion is not likely to impact incidence estimates. Incidences estimates include new injectors recruited from both MMTP and detoxification; including MMTP participants, who might be expected to be at lower risk of HCV acquisition, could lead to underestimates of HCV incidence, both in general, and in comparison with our 2000-2001 data which did not include new injectors recruited from MMTP (Des Jarlais et al., 2005b) We examined trends in HCV prevalence and incidence in temporal relation to changes in combined prevention programming; relationships between these two cannot with certainty be inferred to be causal. Nonetheless, use of historical periods - prior to and after implementation of interventions - is a common method for studying changes over time in epidemics (Des Jarlais et al., 2009a, Des Jarlais et al., 2009b, Des Jarlais et al., 2010, Des Jarlais et al., 2005b, Des Jarlais et al., 2015).
In conclusion, there continues to be a high prevalence of an HCV epidemic among PWID in NYC. New injectors in NYC remain at high risk for HCV. More potent combined prevention, including significant scale up of some combination of MAT, sterile drug injection equipment access, and HCV treatment is urgently needed to control the HCV epidemic among PWID in NYC.
|
|
| |
| |
|
|
|