| |
HCV Genotype 3 Treatment: Daclatasvir+Sofosbuvir, Sofosbuvitr+Peg/Rbv / EASL-Abbvie/Gilead/J&J/Merck/BMS/Achillion/African-Americans/New Drugs
|
| |
| |
Summary from EASL 2015 for Hepatitis C All oral HCV DAA therapy on its way to optimization: still much to learn. - Jurgen K. Rockstroh M.D., Professor of Medicine University of Bonn, Germany (05/22/15)
EASL: Cohort Studies at EASL 2015 - (05/14/15)
EASL: Sofosbuvir Plus Peg-IFN/RBV for 12 Weeks vs Sofosbuvir/RBV for 16 or 24 Weeks in Genotype 3 HCV-Infected Patients and Treatment-Experienced Cirrhotic Patients With Genotype 2 HCV: The BOSON Study - (04/27/15)
APASL: All-Oral 12-Week Combination Treatment With Daclatasvir and Sofosbuvir in Patients Infected With HCV Genotype 3: ALLY-3 Phase 3 Study - (03/18/15)
Clinical Pharm of HIV & Hepatitis Population Viral Kinetic Modeling: SVR Prediction in HCV GT-3 Cirrhotic Patients With 24 Weeks of Daclatasvir + Sofosbuvir Administration - (05/29/15)
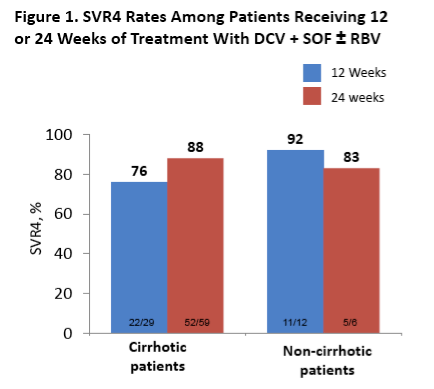
EASL: DACLATASVIR PLUS SOFOSBUVIR WITH OR WITHOUT RIBAVIRIN IN PATIENTS WITH HCV GENOTYPE 3 INFECTION: INTERIM ANALYSIS OF A FRENCH MULTICENTER COMPASSIONATE USE PROGRAM - (04/24/15)
EASL: Abbvie at EASL 2015 - Healthcare Economics, Clinical Trials - (05/11/15)
EASL: Gilead at EASL 2015 - (05/12/15)
EASL: Preclinical Profile of the Pangenotypic HCV NS3/4A Protease Inhibitor GS-9857 - (04/29/15)
EASL: SAFETY AND EFFICACY OF SHORT-DURATION TREATMENT WITH GS-9857 COMBINED WITH SOFOSBUVIR/GS-5816 IN TREATMENT-NAIVE AND DAA-EXPERIENCED GENOTYPE 1 PATIENTS WITH AND WITHOUT CIRRHOSIS - (04/23/15)
Merck at EASL 2015 - (05/22/15)
EASL: Achillion at EASL 2015 - (05/15/15) Just bought by J&J
EASL: J&J at EASL - (05/11/15)
EASL: BMS at EASL - (05/11/15)
EASL: HEALTHCARE ECONOMICS / SVR Improves Health, Mortality / Early HCV Therapy Prefer - (05/14/15)
EASL: 8 HCV DAA Studies in African-Americans - (05/04/15)
EASL: HCV Reinfection in Phase 3 Studies of Sofosbuvir - (04/29/15)
Risk of late relapse or re-infection with Hepatitis C after Sustained Virological Response: meta-analysis of 66 studies in 11,071 patients......http://www.natap.org/2015/CROI/croi_135.htm
EASL: Merck Phase 3 clinical trials evaluating grazoprevir/elbasvir, C-EDGE program, - (04/28/15)
EASL: New HCV Drugs new nukes-NS5A/Protease Inhibitors - (05/08/15)
EASL: ABT530+ABT493 Pangenotypic in Phase 2B - (05/11/15)
EASL: Merck HCV Nuk MK3682 7-Day Monotherapy - (05/05/15)
EASL: ACH-3422, a Novel Nucleotide Prodrug Inhibitor of HCV NS5B Polymerase - (04/31/15) J&J owns
EASL: Preclinical Characterization of AL-335, a Potent Uridine Based Nucleoside Polymerase Inhibitor for the Treatment of Chronic Hepatitis C - (05/05/15) J&J owns
|
|
| |
| |
|
|
|