| |
Continuum of hepatitis C testing and care [in Philadelphia] - HCV Screening/Linkage to Care Programs/funding Needed/Should be the FOCUS
|
| |
| |
Download the PDF here
.....from Jules: as I have been saying without federal and state support but most has to come from federal support we will not be able to begin to address seriously HCV in the USA, this should be a major focus & high profile request to Congress & the White House, but it is NOT.
Routine HCV Screening Project in Philadelphia finds High HCV-Infection Rate, 8.6% infected with HCV: CDC Report - "Identification and Linkage to Care of HCV-Infected Persons in Five Health Centers - Philadelphia, Pennsylvania, 2012-2014" - (05/20/15) .......in Philadelphia ... ."routine HCV testing can be successfully integrated into ambulatory care settings providing services for persons disproportionately affected by HCV infection......overall 8.6% of patients were infected with HCV".......Routine HCV testing and linkage-to-care for persons with current infection were successfully integrated into five federally qualified health centers in Philadelphia, PA. Across the centers, 595 (13.2%) of 4,514 patients tested were HCV-antibody positive and 550 (92.4%) received HCV-RNA testing. Of the 390 (70.9%) with current (chronic) HCV infection, 348 (89.2%) received their results, 304 (78.0%) were referred to and 240 (61.5%) were seen by a provider familiar with HCV care and treatment..... Of the 390 patients with current HCV infection, 25 (6.4%) began antiviral treatment.....Baby Boomers accounted for 62.6% of patients with current HCV infection. Among 352 patients reporting drug use, 205 (58.2%) reported having injected drugs in their lifetime.......overall 8.6% of patients were infected with HCV....reflex confirmatory testing conducted......routine HCV testing can be successfully integrated into ambulatory care settings providing services for persons disproportionately affected by HCV infection..... linkage services included reminder phone calls, public transportation tokens for patients to attend appointments, or patient escorts......providing all current HCV-infected patients with their test results, offering onsite posttest counseling, and referring patients to HCV-care specialists (primary-care providers trained to care for patients infected with HCV, as well as hepatologists or gastroenterologists from one of the local academic medical centers) for medical evaluation
-----------------------
The continuum of hepatitis C testing and care [in Philadelphia; Philadelphia Department of Public Health (PDPH)]
......"fewer than half of city residents likely to be infected with HCV were identified...there is little federal funding in place to provide counseling, testing, and medical referral for HCV, and budget sequestration is likely to reduce this further.....HCV has not received attention commensurate with the burden of disease. Funding allocated toward HCV research is at much lower levels than that earmarked for HIV, in spite of there being 5 times as many individuals infected with HCV.....To promote movement through the continuum of HCV care, state and local health departments need to devise ways to improve surveillance and enhance screening and linkage and retention in HCV care services"......"These findings also show that a higher proportion of young, white individuals who tested positive for HCV Ab were not RNA confirmed."
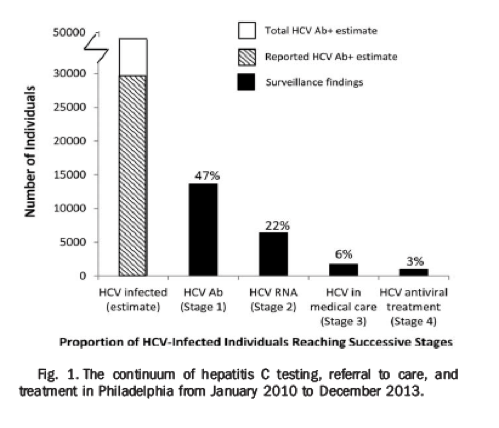
"Philadelphia had approximately 1,584,848 residents between January 2010 and December 2013, and based on seroprevalence estimates from NHANES and high-risk populations, 47,525 (2.9%) would be seropositive for HCV (total HCV Ab+ estimate; Fig. 1). In the study time frame, the Health Department received HCV test results for 39,121, or 61%, of all individuals with a reported HCV test result. Therefore, we would have anticipated receiving a test on 28,990 individuals (61% of 47,525) during the study period (reported HCV Ab+ estimate; Fig. 1).
Positive HCV Ab tests were received for 13,596 of 28,990 (47%) unique individuals during 2010-2013, and only 6,383 (22% of total) of those had infection confirmed by HCV-RNA testing (Fig. 1). The average time between the HCV Ab screening and RNA confirmatory test was 51 days. For individuals whose HCV disease was confirmed, only 1,745 (6% of estimated total morbidity) were in care, defined as having two tests within 6 months or a test ordered by a specialist. Based on interviews with HCV patients first identified during 2013 and their health care providers, 956 (3% of total) individuals were estimated to have received or are currently receiving anti-HCV therapy.'
"The continuum of engagement in HCV care provides a real-life snapshot of how this disease is being addressed in a major U.S. urban center. These findings elucidate how few HCV-infected residents are successfully mobilized from screening through confirmatory testing and into care and treatment.
Understanding and addressing the specific reasons why patients are lost at each stage is critical if public health and clinical care practitioners hope to affect the outcomes of chronic HCV infection......Consistent with national estimates, fewer than half of city residents likely to be infected with HCV were identified by Health Department surveillance......One likely reason for the large prevalence of undiagnosed HCV-infected persons in Philadelphia is lack of testing. Current national HCV screening strategies have had limited success because barriers to testing remain at both the patient and provider levels......inadequate health insurance coverage and limited access to regular health care may limit patients' health-seeking behaviors. Barriers to HCV screening also exist at the provider level. Despite availability of formal recommendations and guidelines on HCV screening, providers' level of knowledge about HCV infection and testing procedures remains low.[32] Fortunately, this study does indicate that the CDC's new HCV baby boomer recommendation is helping to promote routine testing. This may be the direct result of hospital and primary care networks that have implemented systems to facilitate age-based testing, such as routine emergency department screening and electronic reminders for physicians to test a patient born in the target years......Of the HCV Ab+ individuals who were newly reported during the study period, only half had an RNA result reported to the Health Department. Given that an estimated 15% of cases infected with HCV spontaneously clear infection, the subsequent negative lab results would not be reported.[33] Therefore, beyond the expected proportion of negative RNA results in the 2010-2013 data set used for this study, there remains another 33% (approximately 4,490 individuals) who were HCV Ab+ but were not tested for RNA or whose positive RNA result went unreported......Health Department staff are working with local health care providers to raise awareness of HCV reflex confirmatory testing. ......Of the individuals whose disease was confirmed by HCV-RNA testing, 27% showed evidence of being in regular care......Of the individuals whose disease was confirmed by HCV-RNA testing, 27% showed evidence of being in regular care. The definition of in-care used for this study probably overestimates the true number of cases receiving ongoing medical care for HCV infection, given that one of our indicators, serial HCV-RNA tests, may have detected coincidental testing by different providers, not for purposes of disease follow-up. Currently, there is little federal funding in place to provide counseling, testing, and medical referral for HCV, and budget sequestration is likely to reduce this further.[37] This study indicates that only a small proportion of individuals with confirmed HCV infection (15%) have received antiviral treatment."
----------------------
The continuum of hepatitis C testing and care.....'we assessed the HCV CoC in Philadelphia from January 2010 to December 2013 at the population level...using data obtained by the Hepatitis Surveillance Program at the Philadelphia Department of Public Health (PDPH)........For individuals whose HCV disease was confirmed, only 1,745 (6% of estimated total morbidity) were in care, defined as having two tests within 6 months or a test ordered by a specialist. Based on interviews with HCV patients first identified during 2013 and their health care providers, 956 (3% of total) individuals were estimated to have received or are currently receiving anti-HCV therapy.......HCV has not received attention commensurate with the burden of disease. Funding allocated toward HCV research is at much lower levels than that earmarked for HIV, in spite of there being 5 times as many individuals infected with HCV......"
Hepatology March 2015
Kendra Viner, Danica Kuncio, E. Claire Newbern, and Caroline C. Johnson
From the Philadelphia Department of Public Health, Philadelphia, PA
Abstract
A hepatitis C virus (HCV)-infected person will ideally have access to quality health care and move through the HCV continuum of care (CoC) from HCV antibody (Ab) screening, HCV-RNA confirmation, engagement and retention in medical care, and treatment. Unfortunately, studies show that many patients do not progress through this continuum. Because these studies may not be generalizable, we assessed the HCV CoC in Philadelphia from January 2010 to December 2013 at the population level. The expected HCV seroprevalence in Philadelphia during 2010-2013 was calculated by applying National Health and Nutrition Examination Survey prevalences to age-specific census data approximations and published estimates of homeless and incarcerated populations. HCV laboratory results reported to the Philadelphia Department of Public Health and enhanced surveillance data were used to determine where individuals fell on the continuum. HCV CoC was defined as follows: stage 1: HCV Ab screening; stage 2: HCV Ab and RNA testing; stage 3: RNA confirmation and continuing care; and stage 4: RNA confirmation, care, and HCV treatment.
Of approximately 1,584,848 Philadelphia residents, 47,207 (2.9%) were estimated to have HCV. Positive HCV results were received for 13,596 individuals, of whom 6,383 (47%) had a positive HCV-RNA test. Of these, 1,745 (27%) were in care and 956 (15%) had or were currently receiving treatment.
Conclusion: This continuum provides a real-life snapshot of how this disease is being managed in a major U.S. urban center. Many patients are lost at each stage, highlighting the need to raise awareness among health care professionals and at-risk populations about appropriate hepatitis testing, referral, support, and care.
Hepatitis C virus (HCV) is the primary cause of chronic hepatitis disease in the United States, infecting approximately 3.2 million people.[1, 2] A major challenge in the management of HCV is its silent progression from acute to chronic disease. Because acute infection is asymptomatic in 60%-70% of individuals, many only learn that they are HCV positive decades later, after their disease has progressed to cirrhosis, hepatocellular carcinoma (HCC), or liver failure.[3-5] Thus, diagnosis relies heavily on HCV screening of at-risk individuals, including injection drug users, recipients of blood transfusions, solid-organ transplants before 1992 or clotting-factor concentrates made before 1987, patients who ever received long-term hemodialysis; human immunodeficiency virus (HIV)-infected persons, persons with known HCV exposures, children born to chronically infected mothers, and, most recently, adults born during 1945-1965.3,4 Unfortunately, there are still several barriers to successful testing in health care settings.[6-8] Even when patients are successfully screened, many do not receive confirmatory nucleic acid testing (NAT) for HCV RNA.[9] Without NAT results, physicians cannot effectively differentiate between the 15%-25% of people who have resolved infection after exposure to the virus and the remainder with persistent infection.[10] Moreover, screening programs are inadequately comprehensive; recent estimates indicate that 50%-75% of chronically infected people remain unaware of their infection.[11] This has prevented health departments from accurately estimating the burden of HCV infection and disease transmission in the community.
HCV has not received attention commensurate with the burden of disease. Funding allocated toward HCV research is at much lower levels than that earmarked for HIV, in spite of there being 5 times as many individuals infected with HCV.[12] In the past few years, focus has increasingly been brought to HCV through the advent of new interferon-free antiviral regimens with shorter treatment durations and fewer side effects.[13, 14] However, it will likely take time before everyone who is infected is appropriately screened, confirmed, and brought to care. To increase national attention on HCV, the Centers for Disease Control and Prevention (CDC) Division of Viral Hepatitis recently assessed the continuum of engagement in HCV care in the Chronic Hepatitis Cohort Study (CHeCS), which includes 13,000 HCV-infected patients seen within four integrated medical care systems, and the National Health and Nutrition Examination Survey (NHANES), an annual random sample of approximately 5,000 U.S. citizens to measure health and nutritional status through interview and physical examination.[15, 16] Though findings in these studies highlighted gaps at every point in the HCV continuum of care (CoC), the generalizability of their estimates is uncertain.[17] NHANES is known to be impacted by nonresponse bias and excludes populations that are at especially high risk for HCV, including homeless and incarcerated persons.[17] CHeCS only includes HCV-positive individuals, who are insured and often already in care.[18]
To correct for issues in the NHANES and CHeCS data, the current study was undertaken to assess the continuum of engagement in HCV care at a population level, using data obtained by the Hepatitis Surveillance Program at the Philadelphia Department of Public Health (PDPH). Because HCV is a reportable condition in Philadelphia, health care providers and laboratories are mandated to report all positive test results to the Health Department, regardless of risk, insurance status, or testing facility. Findings of this study highlight the points at which patients tend to fall out of the medical system and provide potential explanations for why this occurs.
Materials and Methods
Surveillance
HCV has been a reportable condition in Philadelphia since 2002, and positive HCV laboratory results on Philadelphia residents, including HCV antibody (Ab), RNA, and genotype results, are received by the Health Department from laboratories and health care providers. This study assesses all individuals with first positive HCV test reports from January 2010 through December 2013, the time frame during which the Health Department's hepatitis surveillance data is most robust and there is stability in the annual number of results reported. In January 2011, the Health Department implemented a new surveillance data management system that supports automatic importing of electronic laboratory reporting (ELR), improving both the time for reports to be entered and the data quality (few errors with data entry).
During the study period, approximately 70% of hepatitis reports were received through ELR, 25% through fax or phone reports, and 5% from active case finding. The majority of viral hepatitis laboratory results were entered into the database within 3 days of a test being run, and the completeness of key fields (gender, date of birth, and address) in incoming viral hepatitis reports was 95%-98%. To enhance completeness of HCV reporting during the study period, hepatitis surveillance staff worked with hospital systems that have their own laboratories to assure receipt of any missing HCV test results. As a result of these efforts, an additional 498 HCV test results were entered into the system.
Surveillance data were matched with electronic death certificate data for Philadelphia residents to limit the study population to those not known to have died from any cause. For patients with first test reports received in 2013, the Health Department obtained additional demographic (race/ethnicity), clinical, and risk factor information through patient and/or clinician interviews.
Population Estimates
Using the 2010 U.S. Census population counts for Philadelphia County and 2010-2012 population change estimates (births, deaths, emigration, and immigration) from the American Community Survey (ACS), the number of individuals living in Philadelphia between 2010 and 2013 was estimated.[19, 20] The expected HCV seroprevalence in Philadelphia during this time frame was calculated by applying age-, gender-, and race/ethnicity-specific NHANES rates to 2012 U.S.
Census estimates.[21, 22] A method described by Chak et al. was used to account for populations excluded from NHANES (homeless, incarcerated, and other high-risk populations).[23] HCV seroprevalence estimates for incarcerated populations in the United States range from 12% to 40%, including a seroprevalence estimation in the Philadelphia Prison System (PPS) during 2012.24,25 The average of these estimates (26%) was applied to the annual local estimate of incarcerated individuals (32,000 persons).[26] Similarly, the average of published HCV seroprevalence rates for homeless populations in the United States (37%; range, 22%-53%) was applied to the estimate of homeless persons in Philadelphia (15,473 individuals annually).[23, 27-29] The incarcerated and homeless seroprevalence rates were then used to finalize Philadelphia's city-wide HCV seroprevalence (total HCV Ab+ estimate).
The estimate of individuals living with HCV was limited to individuals who should have received a test during the study period (2010-2013). First, the proportion of the entire HCV surveillance system (reports from 2002 to 2013) represented by those who had their initial HCV Ab+ result in 2010-2013 was calculated. Then, this percentage was applied to the city-wide seroprevalence estimate, assuming that the Health Department should have received a test result for this proportion of seropositive individuals during 2010-2013 (reported HCV Ab+ estimate).
Stages of HCV Testing and Care
The continuum of engagement in HCV care was defined to include the following stages of testing and follow-up: stage 1: patients tested for HCV Ab; stage 2: patients tested for HCV Ab and confirmatory RNA; stage 3: HCV-RNA-confirmed patients who are in medical care; and stage 4: HCV-RNA-confirmed patients who are in care and have received, or are currently receiving, specific antiviral treatment. Reported laboratory results and 2013 surveillance data were used to determine each patient's current stage in the HCV continuum. Based on personal communication with local primary care and hepatitis disease specialists, an HCV patient in care was defined as someone with two or more HCV-RNA tests ordered at least 6 months apart or at least one test ordered by a gastroenterologist, hepatologist, or infectious disease specialist. To estimate the prevalence of treatment, the proportion of individuals who reported treatment among the investigated 2013 cases was applied to the entire cohort.
Analysis
Individuals are represented at each stage through their highest stage reached in the continuum. The average number of cases per month who received their first positive HCV test before and after the August 2012 CDC HCV "baby boomer" testing recommendations were compared using t test analysis. Chi-square analysis was used to compare the demographics of individuals in each stage of the HCV continuum. For this comparison, only the number of patients who reported receiving treatment in 2013 was used.
Institutional Review Board
This study was not submitted to the PDPH Institutional Review Board. Review was not required for this type of study because the research involved the analysis of existing surveillance data.
Results
Philadelphia had approximately 1,584,848 residents between January 2010 and December 2013, and based on seroprevalence estimates from NHANES and high-risk populations, 47,525 (2.9%) would be seropositive for HCV (total HCV Ab+ estimate; Fig. 1). In the study time frame, the Health Department received HCV test results for 39,121, or 61%, of all individuals with a reported HCV test result. Therefore, we would have anticipated receiving a test on 28,990 individuals (61% of 47,525) during the study period (reported HCV Ab+ estimate; Fig. 1).
Positive HCV Ab tests were received for 13,596 of 28,990 (47%) unique individuals during 2010-2013, and only 6,383 (22% of total) of those had infection confirmed by HCV-RNA testing (Fig. 1). The average time between the HCV Ab screening and RNA confirmatory test was 51 days. For individuals whose HCV disease was confirmed, only 1,745 (6% of estimated total morbidity) were in care, defined as having two tests within 6 months or a test ordered by a specialist. Based on interviews with HCV patients first identified during 2013 and their health care providers, 956 (3% of total) individuals were estimated to have received or are currently receiving anti-HCV therapy.
Significantly fewer individuals received their first positive HCV test in the months before the baby boomer testing recommendations than in the months after (Fig. 2; P < 0.001). In addition, a slightly higher proportion of patients tested in the post-baby boomer recommendation era received confirmatory HCV-RNA testing, although this was not statistically significant. The mean time from the HCV Ab screening test to the RNA confirmatory test dropped from 81 days in the pre- to 15 days in the post-baby boomer testing era (P < 0.00001).
The demographic profiles of individuals unique to each of the four stages of the HCV continuum of care were distinct (Table 1). The proportion of male patients was higher among those who had received or who were receiving HCV treatment (stage 4) than those in earlier stages of the continuum (P < 0.001). There was an increase in the proportion of HCV patients who could be classified as baby boomers (45-64 years of age), from stage 1 to 4 of the continuum. Also notable was the large proportion of individuals 44 years of age and younger in the HCV Ab-only group (41%), as compared to individuals in stages 2, 3, and 4 (31%, 22%, and 21%, respectively; P < 0.0001). Although race/ethnicity for most of the patients in this study was not available, differences across the continuum were apparent for those with data. The population in stage 1 was majority white (49%), whereas most people in stages 3 and 4 were black (50% and 45%, respectively). The majority of patients in all four stages of the HCV continuum were U.S. born (82%-88%) with no significant differences found between the groups.
Discussion
The continuum of engagement in HCV care provides a real-life snapshot of how this disease is being addressed in a major U.S. urban center. These findings elucidate how few HCV-infected residents are successfully mobilized from screening through confirmatory testing and into care and treatment.
Understanding and addressing the specific reasons why patients are lost at each stage is critical if public health and clinical care practitioners hope to affect the outcomes of chronic HCV infection.
Consistent with national estimates, fewer than half of city residents likely to be infected with HCV were identified by Health Department surveillance. Under-reporting may explain 3%-5% of the missing cases, a small minority of unidentified morbidity. ELR of positive HCV results from the major reference laboratories, which accounts for the overwhelming proportion of HCV testing in Philadelphia, has assured relatively consistent and accurate data reporting since January 2011. In the years preceding, the Health Department worked actively with labs to maintain timely reporting by fax and hard copy mailing. Data quality, timeliness, and completeness were assured through the creation of monthly quality control and assurance reports. Use of the CLIA-waived OraQuick HCV Rapid Antibody Test (CLIAwaived, San Diego, CA), which received U.S. Food and Drug Administration approval in 2012, might also lead to under-reporting of HCV-infected cases. However, the total number of sites and volume of tests being performed by this method was extremely small (<1%) and unlikely to account for significant numbers. To assure capture of these results moving forward, the Health Department added HCV-positive rapid and point-of-care tests to the reportable laboratory test result list in August 2014. Finally, some missing HCV morbidity might be accounted for by patients who were diagnosed before the study time frame. By limiting to individuals with HCV first reported in the study period, we excluded 1,800 patients with HCV disease who had HCV-RNA testing done during the study time frame. This still accounts for a small proportion of the persons thought to be infected with HCV, but not captured by this study.
One likely reason for the large prevalence of undiagnosed HCV-infected persons in Philadelphia is lack of testing. Current national HCV screening strategies have had limited success because barriers to testing remain at both the patient and provider levels.[30] Patients often do not recognize themselves at risk for infection and their accuracy for recall of risk behaviors, including drug use and sexual encounters, decreases over time.[31] Furthermore, inadequate health insurance coverage and limited access to regular health care may limit patients' health-seeking behaviors. Barriers to HCV screening also exist at the provider level. Despite availability of formal recommendations and guidelines on HCV screening, providers' level of knowledge about HCV infection and testing procedures remains low.[32]Fortunately, this study does indicate that the CDC's new HCV baby boomer recommendation is helping to promote routine testing. This may be the direct result of hospital and primary care networks that have implemented systems to facilitate age-based testing, such as routine emergency department screening and electronic reminders for physicians to test a patient born in the target years.
Of the HCV Ab+ individuals who were newly reported during the study period, only half had an RNA result reported to the Health Department. Given that an estimated 15% of cases infected with HCV spontaneously clear infection, the subsequent negative lab results would not be reported.[33] Therefore, beyond the expected proportion of negative RNA results in the 2010-2013 data set used for this study, there remains another 33% (approximately 4,490 individuals) who were HCV Ab+ but were not tested for RNA or whose positive RNA result went unreported. As already mentioned, it is unlikely that under-reporting of positive lab results is a major contributing factor. These data do indicate an increase in the proportion of individuals who received confirmatory testing subsequent to the baby boomer recommendations, a proportion that is expected to continue increasing in future years. To better understand who receives diagnostic RNA testing after a positive HCV Ab test, the Health Department began requiring that laboratories report all HCV-RNA results, including those with undetectable levels, in August 2014. In addition, Health Department staff are working with local health care providers to raise awareness of HCV reflex confirmatory testing. Reflex confirmatory testing is additional testing automatically performed on an aliquot of the original specimen in response to a positive screening Ab result.[34] Reflex testing saves the patient from needing to return for a second blood draw and reduces loss to follow-up. Indeed, a move toward reflex testing likely accounts for the dramatic drop in the mean number of days from HCV screening to RNA confirmation that was observed for individuals whose testing was conducted after the baby boomer testing recommendations. Other solutions need to be considered for those sites, usually community-based service organizations that use the rapid HCV Ab screening tests but do not have the facilities or resources to run RNA tests. Agencies that order rapid tests should be required to have diagnostic HCV-RNA testing available on-site or written linkage-to-care agreements with a provider who can perform confirmatory testing.[35, 36]
Of the individuals whose disease was confirmed by HCV-RNA testing, 27% showed evidence of being in regular care. The definition of in-care used for this study probably overestimates the true number of cases receiving ongoing medical care for HCV infection, given that one of our indicators, serial HCV-RNA tests, may have detected coincidental testing by different providers, not for purposes of disease follow-up. Currently, there is little federal funding in place to provide counseling, testing, and medical referral for HCV, and budget sequestration is likely to reduce this further.[37] This study indicates that only a small proportion of individuals with confirmed HCV infection (15%) have received antiviral treatment. Given the time, cost, and side effects associated with current HCV drug regimens, many specialists treated only those patients with the most urgent needs and "warehoused" all others until the newest HCV drugs, Olysio (simeprevir) and Sovaldi (sofosbuvir), were approved in November 2013.38 However, though antiviral treatment may be postponed for many patients, regular visits with a medical provider is essential for maintenance of patient health. These appointments assure that the patient's liver health is monitored, including the extent and advancement of fibrosis and the presence of HCC.[39] Physicians are also made aware of new health conditions that may lead to more rapid progression of fibrosis, such as fatty liver disease or hepatitis B virus (HBV) coinfection, and can increase the frequency of visits or initiate treatment accordingly.[39] Equally important is for medical care to assure hepatitis vaccination and reinforce counseling messages on alcohol use and weight management.
To promote movement through the continuum of HCV care, state and local health departments need to devise ways to improve surveillance and enhance screening and linkage and retention in HCV care services. Epidemiological data will aid in understanding why some people may be "stuck" in each stage of the continuum. This analysis indicates that there are significant differences between the age, gender, and race/ethnicity profiles of individuals at each stage of testing and care. It is perhaps not surprising that age is higher for individuals in each stage of the continuum, given that older people have had more time to be exposed, tested, and receive care and because disease severity increases with age. Similarly, African Americans and males may have been more likely to receive antiviral treatment because they are predisposed to developing more severe infection.[40, 41] These findings also show that a higher proportion of young, white individuals who tested positive for HCV Ab were not RNA confirmed. Early evidence suggests that these individuals may represent a new population of injection drug users in Philadelphia. Recognizing epidemiological trends in different jurisdictions can help target local HCV screening and prevention programs, as well as assist in directing resources.
Increasing communication and collaboration between HCV surveillance, prevention, and clinical personnel is critical to improving the HCV CoC. Ideally, surveillance data should be used to directly engage patients in care and inform prevention activities by identifying at-risk populations for screening, vaccination, and education. The Health Department has already initiated several such activities, including the distribution of resources to HCV patients who request additional hepatitis materials during investigation, and the launch of an on-site Twinrix (hepatitis A virus/HBV) vaccine clinic for uninsured and -vaccinated chronic HCV patients identified through surveillance. Surveillance staff also need to utilize relationships that hepatitis prevention coordinators have built with clinicians and stakeholders to help assure effective testing and reporting. However, none of this can happen effectively without strong federal support and greater efforts to promote an implementation science agenda that pulls on surveillance data to execute the CDC's recommendation for routine baby boomer and risk-based screening, as well as enhance the HCV CoC.
|
|
| |
| |
|
|
|