| |
Direct-Acting Antivirals Cure Innate Immunity in Chronic Hepatitis C - EDITORIAL ......"Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function"
|
| |
| |
Download the PDF here
Download the PDF here
Normalizes Natural Killer Cell Function"
"there is evidence that T-cell function remains profoundly altered in most cases after successful IFN-α/ribavirin therapy with T-cell hyporesponsiveness to exogenous stimulation persisting for a long time after virus control, probably as a result of prolonged exposure to viral antigens and consequent exhaustion.....A hierarchy of residual HCV-specific T-cell dysfunction was demonstrated with cytotoxicity and IL-2 production being mostly affected......but NK cell efficiency would be highly desirable, being an essential component of the innate/adaptive immunity cross-talk.....With this in mind, Serti et al1 designed a flawless prospective study to ask whether a purely antiviral regimen could restore NK cell phenotype and function......The data showed that the expression of several NK cell-activating receptors decreased to normal levels within hours of the commencement of treatment. Remarkably, IFN-γ production normalized by week 2 of therapy, whereas markers of cytotoxicity such as expression of the degranulation marker CD107a and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) lagged behind, to normalize by treatment week 8, suggesting a clear hierarchy of restoration of NK cell function.....Interestingly, inhibition of HCV replication decreased concentrations of CXCR3 ligands CXCL10 and CXCL11, which are products of IFN-stimulated genes. More important, normalization of NK function was maintained through the end of treatment, indicating that viral cure is equivalent to an immunologic cure, eliminating the deleterious effects of chronic type I IFN, which constitutes the basis of the NK functional dichotomy illustrated.....Reconstitution of adaptive immunity to HCV, aided by fully functional NK cells, may also have important clinical implications. Indeed, in contrast with impaired HCV-specific CD8+ T cells after pegylated IFN therapy,14 functionally competent HCV-specific CD8+ T cells after IFN-free therapy may confer protection against reinfection......rapid reconstitution of NK cell function after DAA treatment could also contribute to more efficient reversal of severe liver fibrosis and cirrhosis than previously shown with Peg-IFN/ribavirin therapy.23 The pioneer study by Serti et al is leading the way to an exciting new area of translational research in the field......Future avenues of research should address NK cell responses in those few patients who do not respond to DAA treatment, because failure may not entirely depend upon selection of resistance-associated variants.
"Here, we ask whether an IFN-free treatment regimen of daclatasvir (DCV) and asunaprevir (ASV) normalizes innate immune activation and NK cell function.....We show that successful treatment with the DCV/ASV regimen decreases serum levels of the ISG products CXCL10 and CXCL11, and that it decreases STAT1 expression and STAT1 phosphorylation in NK cells. This is associated with changes in the expression of activating and inhibitory receptors on NK cells and a normalization of NK cell function by week 8 of therapy. The results were verified for end-of-treatment (EOT) responders at week 24, indicating that the normalization of NK cell activation and function are maintained in patients who clear HCV......The normalization of NK cell cytokine production was confirmed at the week 24 time point, with significantly increased frequencies of IFNγ, TNFα, and IFNγ/TNFα-producing cells and IFNγ MFI in both the CD56bright (Figure 7E) and the total NK cell populations (Supplementary Figure 2C). Collectively, these results indicate that effective removal of HCV by an IFN-free DAA regimen normalizes both phenotype and function of NK cells.......Although the proliferation of HCV-specific T cells recently has been reported to improve during antiviral therapy with the DAAs faldaprevir and deleobuvir,14 it has not yet been reported to what extent cytokine production of HCV-specific T cells can be recovered. The current data on recovery of IFNγ and TNFα production by NK cells increase hope that this also may occur for T cells. Restoration of cytokine production may result in better immune surveillance and prevention of virologic relapse, which will be an interesting topic to address in future studies."
"The current study shows that DCV/ASV-mediated HCV clearance is associated with a decrease in NK cell activation and a normalization of NK cell cytotoxic effector functions to levels observed in uninfected subjects. The rapid normalization of the NK cell phenotype is associated with a decrease in the percentage of pSTAT1-expressing NK cells and a decrease in the expression level of the ISG STAT1 in NK cells. Collectively, these results confirm that type I IFN-mediated NK cell activation via the IFNα/ß receptor is indeed responsible for the alteration of NK cell function in chronic HCV infection. Thus, the phenotypic and functional NK cell profile that is observed in chronic HCV infection and exacerbated during IFN-based therapy is normalized in IFN-free DAA therapy."
"A rapid decrease in viremia and level of inflammatory cytokines in all patients was associated with decreased activation of intrahepatic and blood NK cells; it was followed by restoration of a normal NK cell phenotype and function by week 8 in patients with undetectable viremia. This normalized NK cell phenotype was maintained until week 24 (end of treatment)......The development of highly effective IFN-free regimens against HCV infection10, 11 provides a unique opportunity to analyze whether and how fast NK cell activation and liver inflammation resolve when HCV replication is blocked. A normalization of NK cell effector functions also would be of interest in the context of adaptive immune responses because HCV-specific T cells are dysfunctional as a result of chronic antigen stimulation in HCV infection.12, 13 Although a recovery of T-cell proliferation recently was reported during treatment of HCV-infected patients with direct-acting antivirals (DAAs),14 it remains unknown whether this translates into a full recovery of effector functions. Past IFN-based treatment regimens were not suitable to answer these questions because IFN not only has an antiviral, but also an immunomodulatory, function.15 IFN-based therapies activate the innate immune response followed by the induction of a refractory state.16 They also exacerbate the functional dichotomy of NK cells toward increased cytotoxic effector functions and reduced IFNγ production.17, 18"
(original article below follows Editorial)
"....rapid reconstitution of NK cell function after DAA treatment could also contribute to more efficient reversal of severe liver fibrosis and cirrhosis than previously shown with Peg-IFN/ribavirin therapy.23 The pioneer study by Serti et al is leading the way to an exciting new area of translational research in the field.........Reconstitution of adaptive immunity to HCV, aided by fully functional NK cells, may also have important clinical implications. Indeed, in contrast with impaired HCV-specific CD8+ T cells after pegylated IFN therapy,14 functionally competent HCV-specific CD8+ T cells after IFN-free therapy may confer protection against reinfection.....This has recently been questioned by work in multiply exposed injecting drug users (IDU) in whom the frequency, magnitude, and breadth of HCV-specific CD4 and CD8 T-cell responses did not differ among exposed uninfected versus incident cases of HCV infection in seronegative IDU.21 Importantly, NK cells were recognized to play a major role in protection from infection, because exposed uninfected subjects had higher frequencies of IFN-γ producing NK cells, and lower frequencies of CD107a expression compared with incident cases of acute hepatitis C....
......Future avenues of research should address NK cell responses in those few patients who do not respond to DAA treatment, because failure may not entirely depend upon selection of resistance-associated variants. Because the kinetics of virus decay are unlikely to differ among next-generation DAAs, work should be focused on difficult to treat genotypes such as genotype 3, for which no specific efficient antiviral is in sight"
Gastroenterology July 2015
MARIO U. MONDELLI
Research Laboratories
Department of Infectious Diseases
Fondazione I.R.C.C.S. Policlinico San Matteo and
Department of Internal Medicine and Therapeutics
University of Pavia
Pavia, Italy
For a number of years, immunologists have strived to understand the mechanisms associated with treatment-induced eradication of hepatitis C virus (HCV). However, interferon (IFN)-α-based therapies did not allow studies examining the role of the virus itself on innate and adaptive immunity to HCV. The study by Serti et al1 published in this issue of Gastroenterology provides a unique opportunity to assess the effect of virus on innate immunity, particularly natural killer (NK) cells, which were previously described by the same authors and other groups to be dysfunctional in this setting (reviewed by Golden-Mason and Rosen2).
HCV is characterized by a high propensity to persist owing to its remarkable skills to evade the host's innate and adaptive immune responses in complicity with permissive intrahepatic microenvironment, which panders to virus replication while limiting immunopathology.3 However, the available direct-acting antivirals (DAAs) have highlighted the vulnerability of HCV that, despite sophisticated countermeasures against host immune surveillance, can now be eradicated within weeks of treatment in most cases,4 whereas the chances of achieving success were significantly lower with the historical pegylated IFN-α/ribavirin combination therapy. The outcome of the latter was uncertain, even accounting for the strongest predictors such as IFN-λ3 polymorphism and viral genotype. Therefore, several investigators sought possible pretreatment and treatment immunologic variables associated with success or failure.
Importantly, those early studies clearly showed that T cells did not play a lead role in viral eradication, because no HCV-specific T-cell changes were observed in patients who did or did not achieve an early virologic response,5 and the overall vigor of the HCV-specific T-cell response decreased during treatment.6 The negative findings on T-cell responses prompted a proliferation of studies on NK cells in the context of hepatitis C treatment, particularly because early work on intrahepatic NK cells isolated from liver explants suggested that innate lymphoid cells were highly prevalent in the liver and constituted about one-half of intrahepatic lymphocytes.7 Moreover, IFN-α is a potent activator of NK cells, thus providing a strong rationale for investigating their role in treatment responses. Two papers from the authors of the present study pointed to the importance of IFN-α-induced early NK cell activation8 and type I IFN signaling9 as a paradigm of response to therapy. Others added further variables associated with responses to IFN-α-based therapies while confirming NK activation as a key factor associated with sustained virologic response (SVR).10 In chronic HCV infection, NK cells are already activated before any exogenous IFN, and are polarized toward cytotoxicity with deficient IFN-γ secretion as a consequence of chronic exposure to endogenous IFN-α.11, 12 This phenomenon is caused by type I IFN-induced phosphorylation of signal transducer and activation of transcription (STAT) 1, which displaces STAT4 at the IFN-α/ß receptor resulting in decreased pSTAT4-dependent IFN-γ production and increased pSTAT1-dependent cytotoxicity (Figure 1).13 The net result is a "functional dichotomy" characterized by enhanced NK cytolytic activity and a simultaneous failure to produce adequate amounts of IFN-γ and tumor necrosis factor (TNF)-α, with consequent inability to eradicate HCV. It follows that IFN-α-based therapies can only accentuate the NK cell functional dichotomy as a result of massive exposure to exogenous IFN-α. Indeed, as a consequence of STAT1 activation, IFN-γ production decreases early in treatment and does not recover for several weeks after IFN-α therapy.8 Whether IFN-γ production is actually restored to normal values after SVR is not known, because the study did not have sufficient follow-up to answer this question.
Figure 1
Continuous exposure to interferon (IFN)-α increases signal transducer and activation of transcription (STAT)1 expression in natural killer (NK) cells and results in increased pSTAT1-dependent cytotoxicity (CD107a expression and tumor necrosis factor-related apoptosis-inducing ligand [TRAIL] expression) and decreased pSTAT4-dependent IFN-γ production, polarizing NK cells toward cytotoxicity. Plasmacytoid dendritic cells (pDC) contribute to IFN-α secretion. Eradication of hepatitis C virus (HCV) by direct-acting antivirals (DAAs) results in normalization of IFN-α concentration in the microenvironment with increased IFN-γ production and reduction of cytotoxicity, whereas this was not possible with IFN-α-based therapies. Increased IFN-γ production by NK cells may protect from reinfection, improve the development of adaptive immunity via reduction of expression of exhaustion markers such as PD-1,20 T-cell immunoreceptor with immunoglobulin (Ig) and ITIM domains (TIGIT) and T-cell immunoglobulin and mucin domain 1 (TIM-1; unpublished data-Burchill MA, Golden-Mason L, Rosen HR. Reversal of T-cell exhaustion in chronic HCV-infected patients by direct-acting antivirals. Hepatology 2014;60:311A. Abstract 224). IFN-γ can also promote apoptosis of hepatic stellate cells (HSC), which are key players in liver fibrogenesis. Cured NK and CD8 T cells can contribute with DAAs to noncytolytic eradication of HCV.
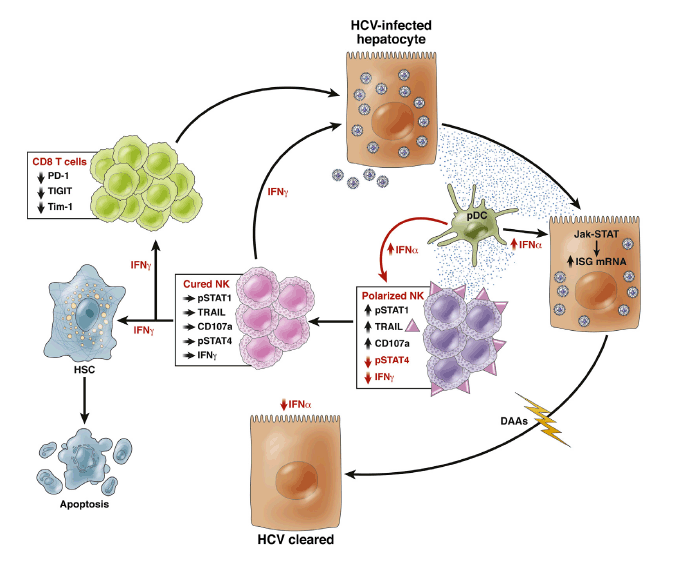
After SVR, one would expect a complete reconstitution of immune cell function. Instead, there is evidence that T-cell function remains profoundly altered in most cases after successful IFN-α/ribavirin therapy with T-cell hyporesponsiveness to exogenous stimulation persisting for a long time after virus control, probably as a result of prolonged exposure to viral antigens and consequent exhaustion.14 A hierarchy of residual HCV-specific T-cell dysfunction was demonstrated with cytotoxicity and IL-2 production being mostly affected. Whether such T-cell functional defects are relevant clinically is unclear presently, although they have the potential to impinge on the efficiency of protection upon reexposure to HCV. NK cell dysfunction is probably not as pervasive,15 but NK cell efficiency would be highly desirable, being an essential component of the innate/adaptive immunity cross-talk.3
With this in mind, Serti et al1 designed a flawless prospective study to ask whether a purely antiviral regimen could restore NK cell phenotype and function. To achieve this, they were able to obtain frozen peripheral blood mononuclear cells from patients with chronic HCV infection receiving a combination of a first wave NS3 protease inhibitor, asunaprevir, and daclatasvir, a first-in-class NS5A inhibitor. The data showed that the expression of several NK cell-activating receptors decreased to normal levels within hours of the commencement of treatment. Remarkably, IFN-γ production normalized by week 2 of therapy, whereas markers of cytotoxicity such as expression of the degranulation marker CD107a and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) lagged behind, to normalize by treatment week 8, suggesting a clear hierarchy of restoration of NK cell function. Interestingly, inhibition of HCV replication decreased concentrations of CXCR3 ligands CXCL10 and CXCL11, which are products of IFN-stimulated genes. More important, normalization of NK function was maintained through the end of treatment, indicating that viral cure is equivalent to an immunologic cure, eliminating the deleterious effects of chronic type I IFN, which constitutes the basis of the NK functional dichotomy illustrated (Figure 1). Indeed, type I IFNs, beside eliciting direct antiviral effects during acute infection, may concurrently boost immunoregulatory responses that prevent robust adaptive immune responses. The recent demonstration that blockade of IFN-I signaling during persistent infection redirects the immune environment to enable control of infection and is associated with enhanced IFN-γ production16, 17 is in keeping with this assumption.
The hitherto unrecognized findings reported by Serti et al1 are instrumental to our understanding of the pathogenesis of hepatitis C and have several relevant translational implications. The fundamental importance of IFN-γ in the control of viral infections has been shown in several studies, including its powerful noncytolytic mechanism of viral clearance from infected hepatocytes.18 Those studies performed in vitro and in animal models were also applicable, for instance, to NK cells from a cohort of patients who developed acute HCV infection in the context of human immunodeficiency virus-1 co-infection.19 Thus, NK cells from patients who spontaneously cleared HCV displayed a greater IFN-γ secretion than those from patients developing chronic infection, and could inhibit HCV replication in the replicon system in vitro. DAA therapy has also been shown to rescue T-cell function from the state of exhaustion induced by co-inhibitory receptors like PD-1.20Interestingly, expression of this molecule gradually decreased under IFN-free treatment, providing direct evidence for a role of chronic exposure to viral antigens in the pathogenesis of T-cell exhaustion (Figure 1). Reconstitution of adaptive immunity to HCV, aided by fully functional NK cells, may also have important clinical implications. Indeed, in contrast with impaired HCV-specific CD8+ T cells after pegylated IFN therapy,14 functionally competent HCV-specific CD8+ T cells after IFN-free therapy may confer protection against reinfection. This has recently been questioned by work in multiply exposed injecting drug users (IDU) in whom the frequency, magnitude, and breadth of HCV-specific CD4 and CD8 T-cell responses did not differ among exposed uninfected versus incident cases of HCV infection in seronegative IDU.21 Importantly, NK cells were recognized to play a major role in protection from infection, because exposed uninfected subjects had higher frequencies of IFN-γ producing NK cells, and lower frequencies of CD107a expression compared with incident cases of acute hepatitis C, suggesting that significant STAT1 phosphorylation did not occur in exposed uninfected individuals and that IFN-γ is a major determinant of protection against HCV infection and reinfection.
Future avenues of research should address NK cell responses in those few patients who do not respond to DAA treatment, because failure may not entirely depend upon selection of resistance-associated variants. Because the kinetics of virus decay are unlikely to differ among next-generation DAAs, work should be focused on difficult to treat genotypes such as genotype 3, for which no specific efficient antiviral is in sight. Another important mission of NK cells is the control of liver fibrosis. Indeed, despite advancements in the field, no treatment options currently exist to halt fibrogenesis, except eradicating the etiological factor. Hepatic stellate cell (HSC) activation during liver injury is a key step in the development of liver fibrosis and previous studies demonstrated that NK cells selectively kill activated HSC. In addition, IFN-γ produced by NK cells induces HSC apoptosis and cell cycle arrest and subsequently inhibits liver fibrosis (Figure 1).22 Thus, rapid reconstitution of NK cell function after DAA treatment could also contribute to more efficient reversal of severe liver fibrosis and cirrhosis than previously shown with Peg-IFN/ribavirin therapy.23 The pioneer study by Serti et al is leading the way to an exciting new area of translational research in the field.
----------------------------------
Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function
Gastroenterology July 2015
Elisavet Serti,1,2 Xenia Chepa-Lotrea,1,2 Yun Ju Kim,2 Meghan Keane,1,2 Nancy Fryzek,2
T. Jake Liang,2 Marc Ghany,2 and Barbara Rehermann1,2
1Immunology Section and 2Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National
Institutes of Health, Department of Health and Human Services, Bethesda, Maryland
"Here, we ask whether an IFN-free treatment regimen of daclatasvir (DCV) and asunaprevir (ASV) normalizes innate immune activation and NK cell function.....We show that successful treatment with the DCV/ASV regimen decreases serum levels of the ISG products CXCL10 and CXCL11, and that it decreases STAT1 expression and STAT1 phosphorylation in NK cells. This is associated with changes in the expression of activating and inhibitory receptors on NK cells and a normalization of NK cell function by week 8 of therapy. The results were verified for end-of-treatment (EOT) responders at week 24, indicating that the normalization of NK cell activation and function are maintained in patients who clear HCV."
"A rapid decrease in viremia and level of inflammatory cytokines in all patients was associated with decreased activation of intrahepatic and blood NK cells; it was followed by restoration of a normal NK cell phenotype and function by week 8 in patients with undetectable viremia. This normalized NK cell phenotype was maintained until week 24 (end of treatment)......The development of highly effective IFN-free regimens against HCV infection10, 11 provides a unique opportunity to analyze whether and how fast NK cell activation and liver inflammation resolve when HCV replication is blocked. A normalization of NK cell effector functions also would be of interest in the context of adaptive immune responses because HCV-specific T cells are dysfunctional as a result of chronic antigen stimulation in HCV infection.12, 13 Although a recovery of T-cell proliferation recently was reported during treatment of HCV-infected patients with direct-acting antivirals (DAAs),14 it remains unknown whether this translates into a full recovery of effector functions. Past IFN-based treatment regimens were not suitable to answer these questions because IFN not only has an antiviral, but also an immunomodulatory, function.15 IFN-based therapies activate the innate immune response followed by the induction of a refractory state.16 They also exacerbate the functional dichotomy of NK cells toward increased cytotoxic effector functions and reduced IFNγ production.17, 18"
Background & Aims
Chronic hepatitis C virus infection activates an intrahepatic immune response, leading to increased expression of interferon (IFN)-stimulated genes and activation of natural killer (NK) cells-the most prevalent innate immune cell in the liver. We investigated whether the elimination of hepatitis C virus with direct-acting antiviral normalizes expression of IFN-stimulated genes and NK cell function.
Methods
We used multicolor flow cytometry to analyze NK cells from the liver and blood of 13 HCV-infected patients who did not respond to treatment with pegylated interferon and ribavirin. Samples were collected before and during IFN-free treatment with daclatasvir and asunaprevir and compared with samples from the blood of 13 healthy individuals (controls). Serum levels of chemokine C-X-C motif ligand (CXCL) 10 or CXCL11 were measured by enzyme-linked immunosorbent assay.
Results
Before treatment, all patients had increased levels of CXCL10 or CXCL11 and a different NK cell phenotype from controls, characterized by increased expression of HLA-DR, NKp46, NKG2A, CD85j, signal transducer and activator of transcription 1 (STAT1), phosphorylated STAT1, and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). NK cells from patients also had increased degranulation and decreased production of IFNγ and tumor necrosis factor α compared with NK cells from controls. Nine patients had an end-of-treatment response (undetectable virus) and 4 had virologic breakthrough between weeks 4 and 12 of therapy. A rapid decrease in viremia and level of inflammatory cytokines in all patients was associated with decreased activation of intrahepatic and blood NK cells; it was followed by restoration of a normal NK cell phenotype and function by week 8 in patients with undetectable viremia. This normalized NK cell phenotype was maintained until week 24 (end of treatment).
Conclusions
Direct-acting antiviral-mediated clearance of HCV is associated with loss of intrahepatic immune activation by IFNα, which is indicated by decreased levels of CXCL10 and CXCL11 and normalization of NK cell phenotype and function.
Materials and Methods
Study Cohort
NK cells were studied in 13 HCV-infected nonresponders to previous PegIFN/RBV therapy before treatment, at days 0 and 1 of treatment, and then at weeks 2, 4, 8, and 24 of a 24-week treatment course with 60 mg DCV once daily and 100 mg ASV twice daily (Bristol-Myers Squibb, New York, NY; ClinicalTrials.gov: NCT01888900). NK cells were studied in 13 uninfected subjects for comparison. Patients underwent paired liver biopsies pretreatment and at either week 2 (n = 5) or week 4 (n = 8) of therapy. Three patients who later experienced virologic breakthrough had another biopsy at week 2 of therapy, the biopsy specimen of the fourth patient with a virologic breakthrough was not studied for NK cell responses. Patients provided written informed consent for research testing under protocols by the Institutional Review Board of National Institute of Diabetes and Digestive and Kidney Diseases/National Institute of Arthritis and Musculoskeletal and Skin Diseases.
Serologic Analyses
Serum HCV-RNA level was quantitated using the Cobas TaqMan real-time polymerase chain reaction (Roche Molecular Diagnostics, Branchburg NJ) with a lower limit of detection of 10 IU/mL and a lower limit of quantification of 25 IU/mL. Serum chemokine C-X-C motif ligand (CXCL) 10 and CXCL11 levels were quantitated using the enzyme-linked immunosorbent assay MAX kit (Biolegend, San Diego, CA) and the Quantikine enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN), respectively.
Lymphocyte Isolation
Peripheral blood mononuclear cells (PBMCs) were separated from heparin-anticoagulated blood on Ficoll-Histopaque (Mediatech, Manassas, VA) density gradients, washed 3 times with phosphate-buffered saline (Mediatech), and cryopreserved in 70% fetal bovine serum (FBS; Serum Source International, Charlotte, NC), 20% RPMI1640 (Mediatech) and 10% dimethyl sulfoxide (Sigma Aldrich, St. Louis, MO). Liver biopsy specimens were homogenized, washed with phosphate-buffered saline, and studied the same day as described later.
NK Cell Analysis
For each patient, cryopreserved PBMCs from week 0 to week 8 were thawed and tested on the same day. A second experiment was performed with cryopreserved samples from week 0 and week 24. Healthy donor PBMCs were included in each experiment. Because the flow cell of the LSRII flow cytometer was replaced between weeks 8 and 24 of the study protocol; the mean fluorescence intensity (MFI) data from the week 24 (EOT) time point cannot be compared with those from the earlier experiment.
NK cell frequency and phenotype
Thawed PBMCs were stained with ethidium monoazide (EMA), anti-CD19-PeCy5, anti-CD3-AlexaFluor700 (both from BD Biosciences, San Jose, CA), and with either anti-CD14-V711 (Biolegend) or anti-CD14-PeCy5 (AbD Serotec, Raleigh, NC) to exclude dead cells, T cells, B cells, and monocytes. NK cells were identified using anti-CD56-PeCy7 (BD Biosciences). Fluorescein isothiocyanate (FITC)-conjugated antibodies against CD122, CXCR3 (R&D Systems), CD69 and HLA-DR (BD Biosciences), PE-conjugated antibodies against tumor-necrosis factor-related apoptosis-inducing ligand (TRAIL), CD300 (BD Biosciences), NKG2A, NKG2D, and NKp44 (Beckman Coulter, Brea, CA), and allophycocyanin (APC)-conjugated antibodies against CD85j (eBiosciences, San Diego, CA), CCR5 (BD Biosciences), NKG2C (R&D Systems), NKp30 and NKp46 (Miltenyi Biotec, Auburn, CA) were added. Liver-infiltrating lymphocytes were stained with anti-TRAIL-PE, anti-CD69-APC/Cy7 (BD Biosciences), and anti-NKp46-APC (Miltenyi Biotec) in addition to EMA and the lineage-specific antibodies described earlier.
NK cell degranulation
Thawed PBMCs were cultured overnight in RPMI1640 with 10% FBS (Serum Source International), 1% penicillin/streptomycin, 2 mmol/L L-glutamine, and 10 mmol/L HEPES (Mediatech). The next day, PBMCs were counted and cultured in the presence of anti-CD107-PE (BD Biosciences) with or without K562 cells (ATCC, Manassas, VA) as described7 without addition of cytokines, and then stained with EMA and lineage-specific antibodies as described earlier.
Cytokine production
Thawed PBMCs were incubated with or without interleukin (IL)12 (0.5 ng/mL; R&D Systems) and IL15 (20 ng/mL; R&D Systems) for 14 hours, followed by the addition of brefeldin A (BD Biosciences) for 4 hours as described.7 Cells then were washed and stained with EMA and the lineage-specific antibodies as described earlier. Cells were washed again, fixed, and permeabilized with the Cytofix/Cytoperm Kit and stained with anti-IFNγ-PE and anti-tumor necrosis factor (TNF)α-APC (all from BD Biosciences).
STAT1/pSTAT1 staining
Thawed PBMCs were rested for 2 hours at 37°C, 5% CO2 in RPMI1640 with 10% FBS (Serum Source International), 1% penicillin/streptomycin, and 2 mmol/L L-glutamine (Mediatech). After fixation with Cytofix (BD Biosciences) for 10 minutes at 37°C and 5% CO2 and subsequent centrifugation, cells were permeabilized with BD Phosflow III (BD Biosciences) for 20 minutes on ice, then washed twice and resuspended in BD Phosflow Buffer (BD Biosciences). All samples were stained with anti-CD56-PE (Beckman Coulter), anti-CD20-PerCP/Cy5.5, anti-CD3-fluorescein isothiocyanate or anti-CD3-APC, and anti-pSTAT1-Alexa488 or anti-STAT1-Alexa647 (all from Biosciences) for 20 minutes at room temperature.
Samples were analyzed on an LSRII flow cytometer using FacsDiva Version 6.1.3 (BD Biosciences) and FlowJo Version 8.8.2 software (Tree Star, Ashland, OR).
Statistical Analysis
D'Agostino and Pearson omnibus normality tests, Wilcoxon signed-rank tests, Mann-Whitney tests, or linear regression analyses were performed with GraphPad Prism 5.0a (GraphPad Software, La Jolla, CA). Two-sided P values less than .05 were considered significant.
Results
Effect of DCV/ASV Therapy on HCV Viremia and Liver Inflammation
All 13 HCV-infected nonresponders to PegIFN/RBV (Supplementary Table 1) experienced a rapid decrease in serum HCV-RNA levels within the first 2 weeks of DCV/ASV therapy (P = .0038) (Figure 1A). Nine patients developed an EOT response, whereas 4 patients had a virologic breakthrough (week 4, n = 1; week 6, n = 2; and week 12, n = 1). Seven of 9 EOT responders achieved a sustained virologic response at 24 weeks after treatment; the remaining had not yet reached week 24 post-treatment. Serum alanine aminotransferase, CXCL10, and CXCL11 levels decreased significantly within the first 8 weeks of DCV/ASV therapy (P = .008, P = .0005, and P = .0007, respectively) (Figure 1B-D).
Effect of DCV/ASV Therapy on NK Cell Activation
The effect of the rapid DAA-mediated decrease in HCV titers on the activation status and function of NK cells was studied by multicolor flow cytometry. Expression of the activation marker HLA-DR, the activating receptor NKp46, and the inhibitory receptors CD85j and NKG2A were higher on NK cells of chronically HCV-infected patients before DAA therapy than on those of uninfected controls (Figure 2). The expression level of HLA-DR, NKp46, CD85j, and NKG2A normalized in patients with undetectable viremia by week 8 of therapy, reaching levels similar to those of NK cells of uninfected controls, and the decrease in expression was greater than in the 3 patients who were viremic at week 8 (Figure 2). Because the latter were offered a rescue protocol with DCV/ASV/PegIFN/RBV, week 8 was the last time point at which to compare patients with and without detectable viremia in this study. Week 8 therefore was chosen to document the effect of a DAA-mediated decrease in viremia on NK cell phenotype and function.
NKp30, NKp44, CD69, NKG2C, NKG2D, CCR5, CD300, and CD122 expression also were assessed. There was a trend of higher NKp30 expression on NK cells of HCV patients compared with those of healthy controls (P = .08), and NKp30 MFI decreased significantly within 8 weeks of DAA therapy (P = .014, not shown). In contrast, the expression level of NKp44, CD69, NKG2C, NKG2D, CCR5, CD300, and CD122 did not differ on NK cells of chronically HCV-infected patients and those of healthy controls and did not change during DCV/ASV therapy (not shown).
Effect of DCV/ASV Therapy on NK Cell Cytotoxicity
NK cells typically show increased cytotoxicity and TRAIL expression but decreased IFNγ production in chronic HCV infection.7, 9 To examine whether the DAA-induced decrease in HCV viremia modulated NK cell effector functions, PBMCs were incubated with major histocompatibility complex class I-negative K562 target cells, and the CD56dim NK cell population was assessed for cell surface expression of CD107a as a read-out for degranulation and cytotoxicity. CD56dim NK cells account for 90% of all NK cells in the circulation and represent the fully mature highly cytotoxic NK cell subset.20 The frequency of CD56dim and CD56bright NK cells did not differ between HCV-infected patients and healthy controls (not shown).
The frequency of CD107a+ cells within the CD56dim NK cell population was higher in chronic HCV patients before therapy than in uninfected subjects (Figure 3A and B, left graphs). A significant decrease in the frequency and expression level of CD107a+ CD56dim NK cells was observed by week 8 of therapy in patients with undetectable viremia (P = .031 and P = .011, respectively) (Figure 3A and B, middle graphs), and the decrease in the percentage of CD107a+ CD56dim NK cells was greater in patients with undetectable viremia than in those who were viremic at week 8 (P = .014) (Figure 3A, right graph). The same pattern was observed in the total CD56+ NK cell population (Supplementary Figure 1A and B).
The frequency of TRAIL+ cells and the TRAIL expression level in the CD56dim NK cell subset also decreased significantly during the first 8 weeks of therapy (P = .012 and P = .004, respectively) (Figure 3C and D, left graphs), and the decrease in the frequency of TRAIL+ cells was greater in patients with undetectable viremia at week 8 than in those who had experienced virologic breakthrough (P = .014) (Figure 3C, right graph). The decrease in TRAIL expression followed this trend (P = .077) (Figure 3D, right graph). Similar results were observed for the CD56bright NK cell subset (not shown), in which TRAIL is highly expressed.21 Specifically, the median frequency of TRAIL+ cells in the CD56bright NK cell subset decreased from 45.7% (interquartile range [IQR], 23.2%-58.5%) before therapy to 22.2% (IQR, 11.4%-47%; P = .027), and median TRAIL expression in CD56bright NK cells decreased from 220 (IQR, 166-233) to 165 (IQR, 32-210; P = .002) during the first 8 weeks of therapy in patients with undetectable viremia (not shown).
Increased NK cell cytotoxicity in chronic HCV infection is thought to be driven by chronic exposure to virus-induced endogenous type I IFN.6, 7, 22 We therefore examined whether the DCV/ASV-induced decrease in HCV titer resulted in decreased expression of STAT1 and pSTAT1. The expression level of STAT1, which itself is the product of an ISG, was significantly higher in CD56dim NK cells of chronic HCV patients than in those of uninfected subjects (P = .003), but decreased by week 8 of therapy (P = .02) (Figure 3E). Again, the absolute decrease in STAT1 expression was greater in NK cells of patients with undetectable viremia than in those with viremia at week 8 of therapy (P = .037) (Figure 3E). Likewise, the frequency of pSTAT1-expressing cells in the CD56dim NK cell subset and the pSTAT1 expression level per cell decreased significantly in patients who had undetectable viremia at week 8 of therapy (P = .002 and P = .006, respectively) (Figure 3F).
Effect of DCV/ASV Therapy on NK Cell Cytokine Production
To assess the capacity of NK cells to produce IFNγ and TNFα, PBMCs were stimulated with IL12 and IL15 in vitro and the CD56bright NK cell subset, which constitutes the main source of NK cell-derived cytokines,22 was studied by flow cytometry. As shown in Figure 4A the percentage of IFNγ-producing cells in the CD56bright NK cell subset and the IFNγ expression level were significantly lower in chronic HCV patients compared with uninfected subjects (P = .007 and P = .008, respectively) and increased within the first 8 weeks of therapy in patients with undetectable viremia (P = .008 and P = .002, respectively) (Figure 5A and B). These observations were consistent with changes in the frequency of TNFα+ cells and IFNγ+/TNFα+ cells in the CD56bright NK cell subset (Figure 4C and D), and were confirmed in the total NK population (Supplementary Figure 1C-F).
Effect of DCV/ASV Therapy on Intrahepatic NK Cells
Next, we examined whether these results extended to the liver. NK cells were studied in paired liver biopsy specimens and blood samples before DCV/ASV and, depending on randomization, at week 2 or 4 of DCV/ASV therapy. No patient had experienced a viral breakthrough at the time point of the second biopsy.
The frequency of CD69+ and NKp46+ cells in the CD56dim NK cell population and the MFI of these markers were significantly higher in the liver than in the blood before DCV/ASV therapy (Figure 5A and B), indicating that NK cells were more activated at the site of infection. In contrast, the frequency of TRAIL+ CD56dim NK cells and the TRAIL expression level per cell did not differ between both compartments (data not shown). The HCV titer before DCV/ASV therapy correlated with the frequency of TRAIL+ CD56bright NK cells in the pretreatment biopsy (P = .03, r = .064 data not shown). Accordingly, the frequency of TRAIL+ CD56dim NK cells in the liver and the TRAIL expression level per cell were lower in the on-treatment biopsy specimen (P = .0005 and P = .048, respectively) (Figure 5C) that was taken after a significant decrease in viremia had occurred (Figure 1A). A similar decrease was observed in the frequency and the expression level of TRAIL+ cells in the intrahepatic CD56bright NK cell population (median frequency, 37% before treatment compared with 13% at weeks 2/4, P = .004; median MFI, 113 before treatment compared with 73 at weeks 2/4, P = .027, not shown).
The frequency of NKp46+ cells and the NKp46 expression level in the intrahepatic CD56dim NK cell population decreased as well (P = .027 and P = .006, respectively) (Figure 5D). Collectively, these data indicate a decrease in NK cell activation and normalization of NK cell function in the blood and in the liver during DAA therapy.
NK Cell Functions Normalize in a Sequential Manner
To investigate how early during DCV/ASV therapy the observed changes in NK cell activation and function occur, we tested PBMCs at 24 hours and at 2 weeks of therapy. All patients were included in this analysis because they showed a significant decrease in viral titer during this time period (Figure 1A). As shown in Figure 6A, expression of the activation marker HLA-DR on the total NK cell population decreased within 24 hours of therapy initiation (P = .005). IFNγ production did not improve significantly during the first 24 hours, but normalized by week 2 of therapy (P = .006) (Figure 6B). None of the other cell surface markers (NKp46, NKG2A, and CD85j), that were expressed differentially on NK cells of HCV-infected patients and healthy controls, changed during the first 2 weeks of treatment (not shown). Markers of NK cell cytotoxicity, such as expression of the degranulation marker CD107 (Figure 6C) and TRAIL (not shown), did not change during the first 2 weeks of therapy.
Normalized NK Cell Function Is Maintained in EOT Responders
Finally, we examined whether the restoration of NK cell phenotype and function were maintained. As shown in Figure 7A, HLA-DR, NKp46, and CD85j expression levels on peripheral blood NK cells were lower at week 24 (EOT) compared with week 0 (P = .008, P = .023, and P = .078, respectively). Interestingly, expression of the chemokine receptor CXCR3 significantly increased from week 0 to week 24 (P = .023) (Figure 7B). This is consistent with a lack of CXCR3 stimulation because the serum concentration of the CXCR3 ligands CXCL10 and CXCL11 had decreased by week 8 of therapy (Figure 1C and D).
Confirming the week 8 data (Figure 3), the frequency of CD107+ and TRAIL+ cells in the CD56dim NK cell subset (P = .022 and P = .023, respectively) (Figure 7C) and in the total NK cell population (P = .016 and P = .039, respectively) (Supplementary Figure 2A) were significantly lower at week 24 than before therapy. Likewise, the frequency of pSTAT1-expressing cells and the expression level of the ISG STAT1 in the CD56dim NK cell subset decreased during the 24-week treatment course (P = .016 and P = .023, respectively) (Figure 7D).
The normalization of NK cell cytokine production was confirmed at the week 24 time point, with significantly increased frequencies of IFNγ, TNFα, and IFNγ/TNFα-producing cells and IFNγ MFI in both the CD56bright (Figure 7E) and the total NK cell populations (Supplementary Figure 2C). Collectively, these results indicate that effective removal of HCV by an IFN-free DAA regimen normalizes both phenotype and function of NK cells.
Discussion
NK cells constitute the main innate immune cell population in the liver. Their activation in chronic HCV infection is associated with increased cytotoxic functions, as assessed by TRAIL expression and degranulation, and with decreased production of the antiviral cytokine IFNγ.7, 9 It previously has been suggested that prolonged exposure to low levels of HCV-induced IFNα is responsible for this phenotype.7 However, IFNα protein has rarely and only at exceedingly low levels been detected in patients23 and chimpanzees24 with HCV infection.
Moreover, a similar NK cell phenotype also has been reported in patients with chronic hepatitis B virus infection,9 a disease that does not induce many ISGs in the liver.25 IFN-free therapy regimens for HCV infection provide a unique opportunity to study the interaction between HCV and the intrahepatic immune system because these regimens rapidly decrease viremia to undetectable levels.
The current study shows that DCV/ASV-mediated HCV clearance is associated with a decrease in NK cell activation and a normalization of NK cell cytotoxic effector functions to levels observed in uninfected subjects. The rapid normalization of the NK cell phenotype is associated with a decrease in the percentage of pSTAT1-expressing NK cells and a decrease in the expression level of the ISG STAT1 in NK cells. Collectively, these results confirm that type I IFN-mediated NK cell activation via the IFNα/ß receptor is indeed responsible for the alteration of NK cell function in chronic HCV infection. Thus, the phenotypic and functional NK cell profile that is observed in chronic HCV infection and exacerbated during IFN-based therapy is normalized in IFN-free DAA therapy. This is of interest in the context of recent findings in a mouse model of lymphocytic choriomeningitis virus (LCMV) induced chronic hepatitis. Blocking of the IFNα/ß receptor in this model increased the number of NK cells and virus-specific CD4 T cells and restored systemic IFNγ levels, thereby inducing LCMV clearance.26, 27
Overall, normalization of NK cell phenotype and function followed a hierarchy. Although a decrease in the expression level of the activation marker HLA-DR was observed within 24 hours of DCV/ASV therapy in parallel to a significant decrease in HCV titer, changes in NK cell function were not observed during this time period. The frequency of IFNγ+ NK cells increased significantly by week 2 of therapy, whereas the decrease in the frequency of CD107a+ NK cells became significant only after week 2. Thus, altered cytokine production by NK cells appears to be more readily reversible than altered cytotoxicity. Importantly, all NK cell read-outs were indistinguishable from those of healthy uninfected subjects by week 8 of DCV/ASV therapy and were confirmed at the week 24 time point in EOT responders.
Because we studied a selected group of patients who all were infected with HCV genotype 1b and nonresponders to previous PegIFN/RBV therapy we performed a detailed characterization of this cohort's NK cell phenotype in comparison with published literature. We confirm earlier studies on increased NKp46 and NKG2A expression on NK cells in HCV infection,7 and in addition report increased expression of the inhibitory receptor CD85j and the activation marker HLA-DR. Increased NKp30 expression in our cohort is consistent with results of De Maria et al,28 who reported higher NKp30 expression on NK cells of HCV-infected patients than those of uninfected controls. In contrast, Nattermann et al29 reported lower NKp30 expression, but in a patient cohort with more diverse HCV genotypes. Importantly, the expression of NKp30, NKp46, HLA-DR, NKG2A, and CD85j normalized during successful IFN-free DAA therapy in our study.
NKp44 and NKG2C expression did not differ between NK cells of HCV-infected patients and healthy donors in our study, which is consistent with published literature.7, 29 Likewise, NKG2D expression did not differ between NK cells of HCV patients and healthy controls, and there was no change in expression during DAA therapy. This is consistent with reports by De Maria28 and Nattermann et al,29 who found no differential expression of NKG2D on NK cells of HCV patients and healthy controls. In contrast, Oliviero et al9 reported increased NKG2D levels whereas Dessouki et al30 reported decreased NKG2D levels in HCV infection. However, the patient cohort studied by Oliviero et al9 included responders to IFN-based therapy, and the patients were infected with diverse genotypes and had lower HCV viremia and alanine aminotransferase values than in our study. Dessouki et al30 studied only the frequency of NKG2D+ NK cells and not the NKG2D expression level.
We propose that the restoration of NK cell function is the result of rapid removal of the HCV and its associated IFN signature, also should be seen with other direct-acting antivirals. It also should extend to other HCV genotypes, if they are not associated with an increased incidence of breakthrough or relapse. The restoration of NK cell function, in particular the normalization of suppressed IFNγ and TNFα production, may be relevant for studies on adaptive immune responses. Similar to NK cells, HCV-specific T cells show impaired cytokine production in chronic HCV infection.12, 13 Although the proliferation of HCV-specific T cells recently has been reported to improve during antiviral therapy with the DAAs faldaprevir and deleobuvir,14 it has not yet been reported to what extent cytokine production of HCV-specific T cells can be recovered. The current data on recovery of IFNγ and TNFα production by NK cells increase hope that this also may occur for T cells. Restoration of cytokine production may result in better immune surveillance and prevention of virologic relapse, which will be an interesting topic to address in future studies.
|
|
| |
| |
|
|
|